The absolute contraindications of spirometry are patients with recent eye surgery, open heart surgery, laparotomy, stroke, cardiac arrest, myocardial infarction, pneumothorax, retinal detachment, and aortic aneurysm within 3 months. Other contraindications are patients with hyperventilation, diseases in which maximal ventilatory effort can be problematic (Moyamoya disease, repetitive spontaneous pneumothorax) or those with current respiratory infection, such as tuberculosis or their families were exposed to such infection, those with massive hemoptysis in the past 1 month, and those whose systolic blood pressure exceeds 200 mm Hg or diastolic blood pressure exceeds 140 mm Hg.
The relative contraindications of spirometry are patients with experience of urinary incontinence, those with chest or abdominal pain, those who have pain inside the mouth or on the face when biting the mouth piece, and those with dementia or decreased consciousness.
3. Interpretation
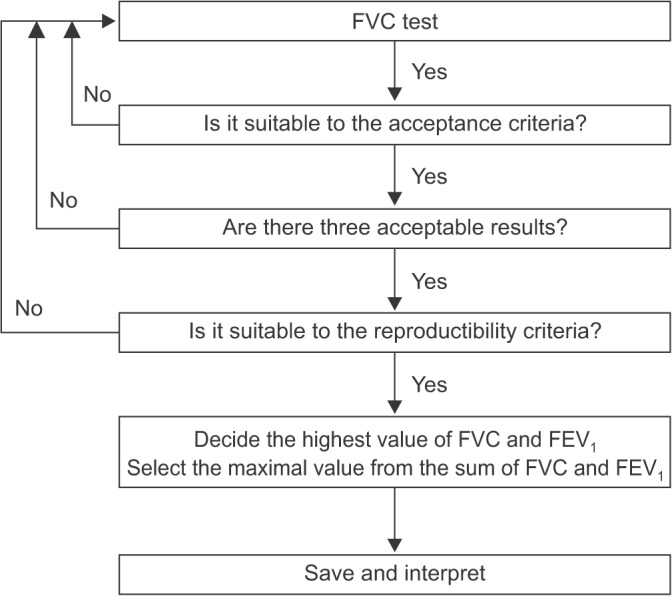
Interpretation of spirometry should be clear, concise, and informative, but a simple description as normal or abnormal only is not helpful. Additionally, this test should have optimal quality before interpretation of spirometry.
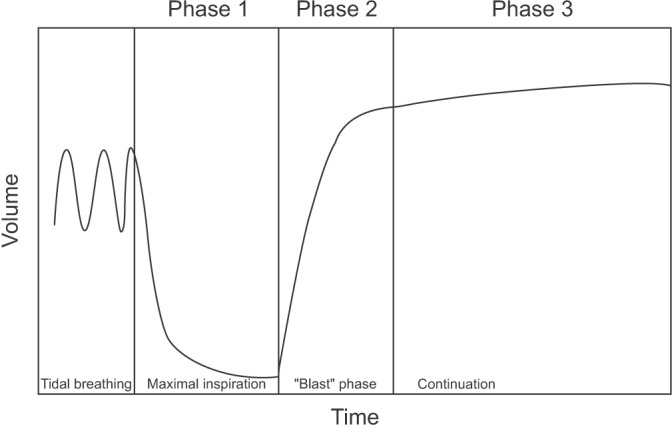
Even when the result of the test is not appropriate, useful information about the patient may be provided. The interpreter should be aware of the potential problem of the test performed and the possible inaccuracy of the interpretation. Most inappropriately performed spirometry tests are due to test subject-related factors. Therefore, an appropriate test performance can be ensured by carefully observing the subjects and the volume-time curve, as well as the flow-volume curve, during the test. Additionally, when interpreting spirometry results, they should be considered together with the clinical findings of symptoms. Those include respiratory symptoms, such as cough and dyspnea, past medical history, smoking history, examination results, and chest radiology reports.
Generally, spirometry provides the following important information. (1) FVC is defined as the total expiratory volume from one time of a maximally forced expiration maneuver. (2) FEV1 is defined as expiratory volume that has been exhaled at the end of the first second of a maximally forced expiration maneuver. (3) FEV1/FVC (FEV1 ratio) is defined as the ratio of FEV1 and FVC and is also expressed as a percentage. (4) Forced expiratory flow during the middle half of FVC (25%–75%) is defined as the average forced expiratory flow of 50% during the middle half of the test, except for the measurement of 25% at the beginning of the test and at the end of FVC. (5) Peak expiratory flow is defined as the maximal flow that is achieved during forced expiration. (6) Maximal voluntary volume, which is known as maximum voluntary ventilation, is a measure of the maximum volume of air that can be inhaled and exhaled within 1 minute by the maximal forced voluntary breathing of a patient. This is performed over a 12 or 15 second time period before being extrapolated to a value for 1 minute.
Interpretation of spirometry requires comparison between values that are measured from subjects and those that are measured in normal healthy subjects. Normal values of spirometry may differ depending on the physical requirements of the subjects, such as the race, sex, age, height and weight, measurement conditions, statistical methods, and socioeconomic or epidemiological requirements. Korea uses the Morris Asian Quanjer equation, described by Morris et al. in 1971
9, or normal predictive values of spirometry in the Korean population, which were based on 2001 National Health and Nutrition Examination Survey
10.
Reference values vary by each proposed guideline for diagnostic criterion for ventilatory defects. A fixed value as the reference value can be a standard indicator for simple, independent clinical application. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define COPD as FEV
1/FVC less than 0.7 from a post-bronchodilator test
11. However, when considering FEV
1/FVC less than 0.7 as the baseline for obstructive ventilatory defects, because FEV
1/FVC decreases as age increases, false-positivity is likely to occur. False-positivity can result in the wrong diagnosis for obstructive pulmonary disease in healthy, older subjects who have never been exposed to risk
12. When the same criteria are applied to young subjects, they may be diagnosed as being normal, even though they have obstructive pulmonary disease
13. Therefore, a reference point of obstructive lung disease in young subjects, if they have clinical symptoms and a medical history, can be increased to FEV
1/FVC <0.75–0.80
13.
To overcome these problems, the American Thoracic Society and the European Respiratory Society defined the value corresponding to the lower fifth percentile of spirometry in the same age normal group that is 95 percentile method as the criteria of ventilation disorders as the lower limit of normal
14. The American Thoracic Society and European Respiratory Society also recommend using vital capacity (VC) instead of FVC in the interpretation of spirometry
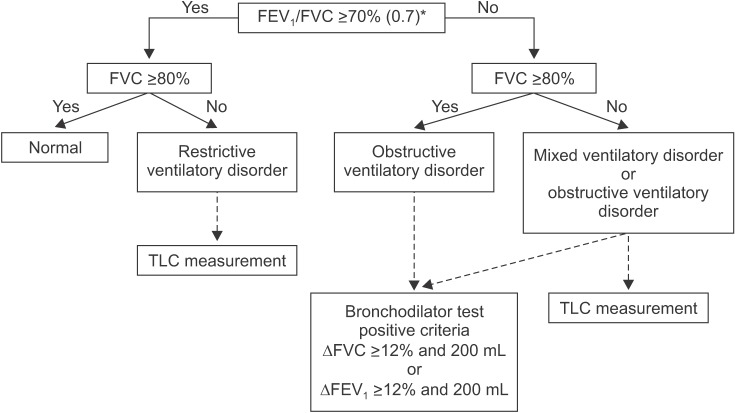
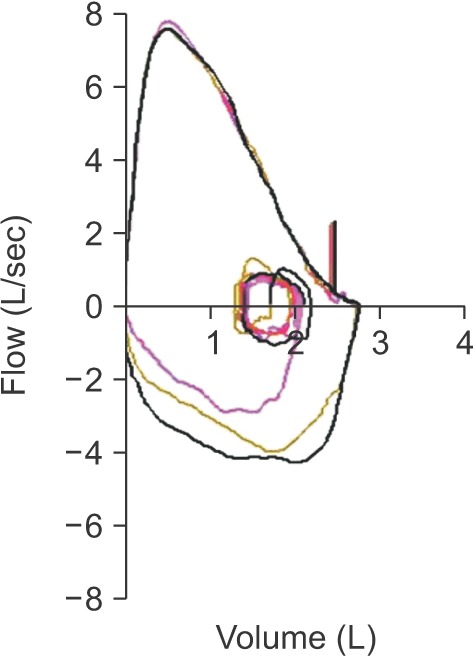
14. VC is generally measured as greater than FVC. Additionally, VC is more accurate than FVC when the airway is flexible, such as in the case of emphysema, because it can diagnose airway obstruction with more certainty. However, our guideline adopts FVC instead of VC for interpretation in most other guidelines because the measurement of VC at spirometer is impossible. Ventilatory disorders can be divided into three main types. Obstructive ventilatory disorders occur when maximum flow rate is reduced compared with maximal volume, FVC, during a forced exhalation maneuver due to airway obstruction. When FEV
1/FVC is less than 0.7 in spirometry, it is interpreted as having obstructive ventilatory defects (
Figure 4). For the initial stage of obstructive pulmonary disease, initial forced expiratory flow remains normal, but late forced expiratory flow is decreased. Therefore, in this situation, FEV
1/FVC may be maintained as normal. FEV
1 is reduced when obstructive pulmonary disease further progresses causing severe airway obstruction. Representative diseases of obstructive pulmonary disease include asthma and COPD.
Restrictive ventilation disorders are characterized by a reduced TLC with normal FEV
1/FVC (
Figure 4). Spirometry can confirm that FVC is reduced, while FEV
1 may be reduced secondarily to a reduction in FVC, or relatively maintains as normal. As a result, FEV
1/FVC is normal or may increase slightly. FVC can also be reduced when a subject does not perform a full inspiratory–expiratory effort, whereas TLC is maintained as normal. Therefore, when restrictive ventilatory disorders are suspected when FVC is reduced as shown by spirometry, measurement of TLC is necessary for accurate diagnosis
14. Pulmonary fibrosis, chest wall disease, or neuromuscular diseases can show restrictive ventilatory defects. Measurement of diffusing capacity is necessary to differentiate pulmonary parenchyma disease, chest wall disease, and neuromuscular disease.
Mixed ventilation disorders occur when obstructive and restrictive ventilatory defects occur together, in which FEV
1/FVC decreases, as well as TLC. Obstructive or restrictive ventilatory defects can show a decrease in FVC from spirometry. Therefore, measurement of TLC is required to accurately determine the concurrence of restrictive and obstructive ventilatory defects (
Figure 4). Mixed ventilatory defects are observed in fibrothorax accompanied by tuberculosis sequelae-induced airway obstruction or in smokers with COPD accompanied by pulmonary fibrosis.
PFT values in classification of lung function impairment show an association with daily life performance, severity of disease, and prognosis of patients with respiratory diseases. However, these values cannot predict exact symptoms or prognosis of individuals. When residual volume is increased, which leads to a reduction in FVC in obstructive pulmonary diseases, FEV1/FVC may not decrease at all. Therefore, FEV1/FVC helps to determine the presence of airway obstruction, but is not appropriate for identifying the severity of obstructive ventilation disorders.
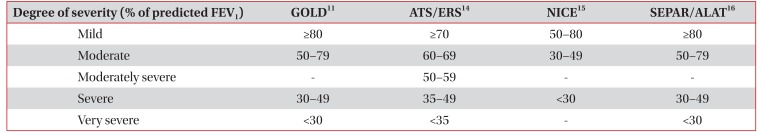
To determine the severity of airway obstruction, a percentage of the predicted normal value of FEV
1 is used. The severity of obstructive pulmonary diseases is shown in
Table 211141516. The severity of restrictive ventilation disorders is related to the degree of pulmonary parenchyma disease and neuromuscular disease, but it cannot reflect the condition of the patient. Classification of the severity of restrictive ventilation disorders uses VC and TLC
17. However, the American Thoracic Society and European Respiratory Society recommended using FEV
1 for classification of the severity of all ventilation impairment, including restrictive ventilation disorders, in 2005 (
Table 3).
It can be regarded as respiratory failure in severe chronic lung diseases if the patient has been diagnosed as an underlying pulmonary disease for more than 1 year and if the lung disability of the patient is fixed in spite of sufficient treatment over the past 2 months.
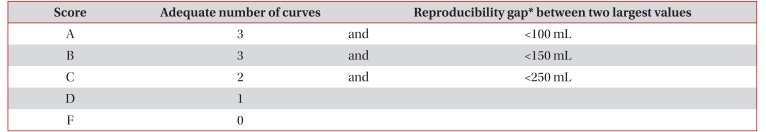
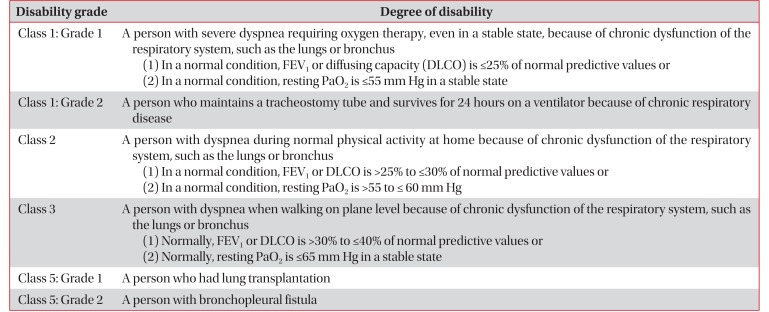
Table 41417 shows the Criteria for Pulmonary Disability-Grading system provided by the Ministry of Health and Welfare in 2015
18.
The descending curve of the normal air flow–volume curve forms a relatively straight line after reaching a peak. However, in obstructive pulmonary disease, the maximal forced expiratory flow rate is reduced and the descending curve shifts sharply downward with an increase in downward concavity after reaching the peak. Lung capacity is mainly reduced, but the airflow rate does not decrease much in restrictive ventilation disorders. Therefore, the flow-volume curve forms a tall and narrow shape. The maximal forced expiratory flow rate is relatively maintained, and the descending curve forms a straight line with a steep slope.
If a plateau is formed from inhalation or exhalation in a flow-volume curve, stenosis of the upper airway or central airway is suspected. If this condition is clinically suspected, its progress should be observed and the airway should be evaluated. Also, further evaluation including imaging study and bronchoscopy is recommended in this situation.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download