Abstract
Raoultella species are gram-negative, non-motile, aerobic bacilli that are primarily considered as environmental bacteria. Raoultella planticola is reportedly a rare cause of human infections. Also, the definite pathological mechanism of Raoultella planticola is currently unknown. We report a case of pneumonia caused by Raoultella planticola.
Raoultella planticola is a gram-negative, non-motile, aerobic bacillus that is primarily considered as environmental bacteria1. R. planticola was previously described as Klebsiella planticola and K. trevisanii, were combined into one species in 1986, i.e., K. planticola, based on DNA-DNA homology. In 2001, K. planticola was renamed R. planticola based on 16S rRNA and rpoB gene sequencing2.
R. planticola does not typically cause invasive infections in humans; furthermore, current literature suggests that the bacteria is a rare cause of human infections. In the present report, we describe a patient with pneumonia due to primary infection by R. planticola.
A 58-year-old man visited our hospital with cough, purulent sputum, and dyspnea since a month prior. The patient presented no medical history except a 30 pack-year smoking history.
On admission, the patient's vital signs were as follows: blood pressure 100/70 mm Hg, pulse rate 92 beats per minute, body temperature 37.4℃, and respiratory rate 35 breaths per minute. Auscultation of lungs revealed coarse breathing sounds with crackle and wheezing on both lower lung fields. The oxygen saturation was 94%, pH 7.48, PaO2 63.0 mm Hg, and PaCO2 32.0 mm Hg while the patient was breathing ambient air. A complete blood count showed a white blood cell (WBC) count of 6,920/mm3 (neutrophils 70.9%, lymphocytes 21.9%, monocytes 20.9%, and eosinophils 9.8%), a hemoglobin of 12.4 g/dL, and platelets 198,000/mm3. Serum chemistry showed blood urea nitrogen 18.6 mg/dL, creatinine 1.0 mg/dL, aspartate aminotransferase 158 IU/L, alanine aminotransferase 439 IU/L, alkaline phosphatase level of 386 IU/L, and a C-reactive protein 7.0 mg/dL. The following day, WBC increased to 19,220/mm3 with neutrophil 91.4%; and C-reactive protein increased to 8.5 mg/dL. An electrocardiogram revealed normal sinus rhythm and cardiac enzyme within was normal range.
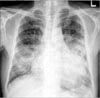
Plain chest radiography on admission showed cardiomegaly and consolidation on both lung fields (Figure 1). Contrast-enhanced chest computed tomography demonstrated diffuse ground glass opacity and consolidation in both lungs. Underlying emphysematous and bullous changes were also noted predominantly in the lower lobes (Figure 2). An echocardiography showed an ejection fraction of 10% and severe left ventricular systolic dysfunction with no regional wall motion abnormality.
Initially, we suspected bacterial pneumonia and heart failure consequent to pulmonary edema. Empirically, levofloxacin, piperacillin-tazobactam, and diuretics were started intravenously. At 7 days follow-up after admission, 2 sputum cultures showed R. planticola with showed sensitivity to most antibiotics except for ampicillin and ciprofloxacin; no different bacteria were detected. Blood cultures were sterile. We diagnosed a pneumonia caused by R. planticola. We stopped levofloxacin based on the sputum result and continued piperacillin-tazobactam treatment.
Seven days after hospitalization, dyspnea had improved and cough and sputum production was reduced. Fifteen days after admission, laboratory analysis was as follows: a WBC count of 5,420/mm3 (neutrophils 55.9%) and C-reactive protein 0.26 mg/dL. At 3 weeks follow-up after admission, chest radiography showed decrease in both ground glass opacity and consolidation in both lung fields; in addition, contrast-enhanced chest computed tomography showed improvement of pneumonia (Figure 3). The patient continued antibiotic therapy for 4 weeks and was discharged. Since discharge, he has been followed up at an outpatient clinic without evidence of recurrence.
R. planticola is an aquatic, botanical and soil organism that does not typically cause invasive infections in humans. Raoultella species produce histidine decarboxylase and have been implicated in scombroid (histamine) fish poisoning, but the clinical significance of this organism in humans has not been characterized3.
R. planticola is found to have similar pathogenetic features as Klebsiella species. Klebsiella spp. are associated with severe infections in hospitalized and immunocompromised patients, including bacteremias, pneumonias, and urinary tract infections, and have been estimated to cause between 3% and 7% of all nosocomial infections2. In 2000, Podschun et al. evaluated the common virulence factors between R. planticola and the Klebsiella spp.2 Specifically, both K. pneumoniae and R. planticola exhibit type 1 fimbriae and mannose-sensitive hemagglutination. Klebsiella spp. have the ability to resist the bactericidal effects of human serum, a feature that is shared by R. planticola2.
R. planticola is a rare cause of human infections and only 18 cases have been reported worldwide. The first case report was described by Freney et al.4 in Lyon, France, of 69-year-old patient with R. planticola bacteremia who was admitted to an intensive care unit 9 days following a mitral valve replacement.
Extensive literature search, indicated a total of eighteen cases of R. planticola infections, including infections with K. planticola and K. trevisanii (Table 1). This case reports included one cholecystitis1, two urinary tract infections25, seven bacteremias24678, one central line infection3, three soft tissue infections91011, one pancreatitis12, two cholangitis1314, and one pneumonia15. Of the eighteen cases, four case patients died and the others reportedly recovered. This is the first case in Korea of community-acquired pneumonia with R. planticola.
Previous reports, suggested the potential risk factors as invasive medical procedures, and trauma with potential soil contamination, with significant comorbidities except for 1 case1. A rare case of cholecystitis caused by R. planticola was reported in 2012. Interestingly, the patient in this report did not have any of these risk factors. In our case, distinguishing between pathogen and colonization was difficult; however, except for R. planticola, no any other bacteria grew in sputum culture. Furthermore, the sputum grade was group 5 (leukocyte >25/low power field [LPF], epithelial cell <10/LPF) and a predominant gram-negative bacilli by Gram stain, and pathogen quantity was moderate to heavy. Hence, it is likely that R. planticola developed pneumonia due to pathogenic action.
The natural susceptibility of 221 Klebsiella strains to 71 antibiotics was examined. R. planticola were sensitive to different penicillins except oxacillin, amoxicillin. Also, R. planticola were sensitive to all cephalosporins and carbapenems9. Recently, the emergence of carbapenem resistance R. planticola has been sporadically reported515. The known mechanism of carbapenem resistance in R. planticola is production of carbapenemases, including class A β-lactamase (KPC), class B metal-β-lactamase (IMP-8, NDM-1), and class D β-lactamase (OXA-48). It is notable that blaKPC, blaIMP-8, and blaNDM-1 were usually located on plasmids or transposons, suggesting possible gene exchange between R. planticola and other Enterobacteriaceae such as K. pneumoniae15. Although most of the R. planticola isolates published in literature are susceptible to carbapenems5, a study on the antimicrobial resistance of R. planticola is also required.
In conclusion, R. planticola is an environmental bacterium that can cause serious infections in humans. Commonly, it does not cause human infections in the normal state, but human infections are in invasive medical procedures, trauma with potential soil contamination, significant comorbidities or with the decline of immunization state. Our case was meaningful in that community-acquired R. planticola infection was developed in a normal state. Elucidating the mechanism of action of R. planticola in human infections is difficult. However, as new laboratory techniques become more common, the mechanism of action of R. planticola in human infections is likely to be revealed.
Figures and Tables
Figure 2
Chest computed tomography demonstrated diffuse ground glass opacity and consolidation in both lungs (A). Underlying emphysematous and bullous changes are also noted predominantly in both lower lobes (B).
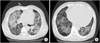
Figure 3
Follow-up, chest radiography (A) and computed tomography (B) showed improvement of ground glass opacity and consolidation in both lung fields.
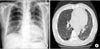
Table 1
Summary of the reported cases of Raoultella planticola infections

| Authors | Age/Sex | Clinical | Treatment | Outcome |
|---|---|---|---|---|
| Teo et al.1 | 62 yr/F | Cholecystitis | Amoxicillin/clavulanic acid | Recovered |
| Olson et al.2 | 89 yr/M | Cystitis | Ciprofloxacin | Recovered |
| Olson et al.2 | 57 yr/M | Bacteremia | Ceftriaxone | Recovered |
| Olson et al.2 | 69 yr/M | Bacteremia | Ceftriaxone, tobramycin | Recovered |
| Nada and Areej3 | 15 mo/F | Central line infection | Ampicillin, gentamicin, metronidazole | Recovered |
| Freney et al.4 | 47 yr/M | Bacteremia | Cefotaxime, tobramycin | Recovered |
| Gangcuangco et al.5 | 92 yr/F | UTI | Ceftriaxone | Recovered |
| Castanheira et al.6 | 83 yr/F | Pneumonia, bacteremia | Carbapenem | Died |
| Castanheira et al.6 | 64 yr/M | Bacteremia | Imipenem/doxycycline | Died |
| Hu et al.7 | 59 yr/M | Bacteremia | Piperacillin-tazobactam | Recovered |
| Puerta-Fernandez et al.8 | 63 yr/M | Bacteremia | Cefotaxime | Recovered |
| Kim et al.9 | 66 yr/M | Necrotizing fasciitis | Ceftriaxone, levofloxacin | Recovered |
| Wolcott et al.10 | 66 yr/M | Surgical site infection | Etrapenem | Recovered |
| O'Connell et al.11 | 30 yr/F | Cellulitis | Clindamycin, ciprofloxacin | Recovered |
| Alves et al.12 | 45 yr/M | Pancreatitis | Imipemen, amikacin | Recovered |
| Lee et al.13 | 75 yr/M | Cholangitis | Cefotaxime, metronidazole | Died |
| Yokota et al.14 | 65 yr/M | Cholangitis, bacteremia | Meropenem | Recovered |
| Xu et al.15 | 60 yr/M | Pneumonia | Imipenem/cilastatin | Died |
| Present case | 58 yr/M | Pneumonia | Piperacillin-tazobactam, levofloxacin | Recovered |
References
1. Teo I, Wild J, Ray S, Chadwick D. A rare case of cholecystitis caused by Raoultella planticola. Case Rep Med. 2012; 2012:601641.
2. Olson DS Jr, Asare K, Lyons M, Hofinger DM. A novel case of Raoultella planticola urinary tract infection. Infection. 2013; 41:259–261.
3. Nada B, Areej M. Raoultella planticola, a central venous line exit site infection. J Taibah Univ Med Sci. 2014; 9:158–160.
4. Freney J, Fleurette J, Gruer LD, Desmonceaux M, Gavini F, Leclerc H. Klebsiella trevisanii colonisation and septicaemia. Lancet. 1984; 1:909.
5. Gangcuangco LM, Saul ZK. A novel case of Raoultella planticola urinary tract infection in a female: comment on 'Nosocomial pneumonia caused by carbapenem-resistant Raoultella planticola : a case report and literature review'. Infection. 2015; 43:621–622.
6. Castanheira M, Deshpande LM, DiPersio JR, Kang J, Weinstein MP, Jones RN. First descriptions of blaKPC in Raoultella spp. (R. planticola and R. ornithinolytica): report from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol. 2009; 47:4129–4130.
7. Hu AY, Leslie KA, Baskette J, Elsayed S. Raoultella planticola bacteraemia. J Med Microbiol. 2012; 61(Pt 10):1488–1489.
8. Puerta-Fernandez S, Miralles-Linares F, Sanchez-Simonet MV, Bernal-Lopez MR, Gomez-Huelgas R. Raoultella planticola bacteraemia secondary to gastroenteritis. Clin Microbiol Infect. 2013; 19:E236–E237.
9. Kim SH, Roh KH, Yoon YK, Kang DO, Lee DW, Kim MJ, et al. Necrotizing fasciitis involving the chest and abdominal wall caused by Raoultella planticola. BMC Infect Dis. 2012; 12:59.
10. Wolcott R, Dowd S. Molecular diagnosis of Raoultella planticola infection of a surgical site. J Wound Care. 2010; 19:329–332.
11. O' Connell K, Kelly J, Niriain U. A rare case of soft-tissue infection caused by Raoultella planticola. Case Rep Med. 2010; 2010:pii: 134086.
12. Alves MS, Riley LW, Moreira BM. A case of severe pancreatitis complicated by Raoultella planticola infection. J Med Microbiol. 2007; 56(Pt 5):696–698.
13. Lee JH, Choi WS, Kang SH, Yoon DW, Park DW, Koo JS, et al. A case of severe cholangitis caused by Raoultella planticola in a patient with pancreatic cancer. Infect Chemother. 2012; 44:210–212.
14. Yokota K, Gomi H, Miura Y, Sugano K, Morisawa Y. Cholangitis with septic shock caused by Raoultella planticola. J Med Microbiol. 2012; 61(Pt 3):446–449.
15. Xu M, Xie W, Fu Y, Zhou H, Zhou J. Nosocomial pneumonia caused by carbapenem-resistant Raoultella planticola : a case report and literature review. Infection. 2015; 43:245–248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download