Abstract
Background
Specific immunoglobulin E (IgE) sensitization to staphylococcal enterotoxin (SE) has been recently considered to be related to allergic disease, including asthma. Despite studies on specific IgE (sIgE) to SE and its relationship to asthma diagnosis and severity, the association of sIgE to SE with airway hyperresponsiveness (AHR) remains unclear.
Methods
We enrolled 81 asthma patients admitted to the Severance Hospital in Korea from March 1, 2013, to February 28, 2015 and retrospectively reviewed the electronic medical records of the enrolled subjects. The serum levels of sIgE to SE (A/B) of all subjects was measured using the ImmunoCAP 250 (Phadia) system with SE-sIgE positive defined as >0.10 kU/mL.
Results
The SE-sIgE level was not significantly correlated with asthma severity (forced expiratory volume in 1 second [FEV1], FEV1/forced vital capacity, sputum eosinophils, and serum eosinophils), whereas the SE-sIgE level in patients with positive AHR (mean±standard error of the mean, 0.606±0.273 kU/mL) was significantly higher than that in patients with negative AHR (0.062±0.015 kU/mL, p=0.034). In regression analysis, SE sensitization (sIgE to SE ≥0.010 kU/mL) was a significant risk factor for AHR, after adjustment for age, sex, FEV1, and sputum eosinophils (odds ratio, 7.090; 95% confidence interval, 1.180–42.600; p=0.032). Prevalence of SE sensitization was higher in patients with allergic rhinitis and non-atopic asthma patients, as compared to patients without allergic rhinitis and atopic asthma patients, respectively, but without statistical significance.
Staphylococcus aureus is a human commensal microorganism, and frequent colonizer of airways and skin. S. aureus releases a wide range of enterotoxins1. While bacterial infection commonly stimulates the innate immune system, staphylococcal enterotoxin (SE) can act as an antigen, in particular, a superantigen2. SE binds to the variable β-chain of the T-cell receptor, independent of the antigen-specific groove, so that it is called a "superantigen." This allows the polyclonal activation of T cells and also B cells, resulting in the formation of specific immunoglobulin E (sIgE) to SE, called "SE sensitization"234. These superantigenic properties of SE are considered to have a role in the pathophysiology of allergic disease.
The first allergic disease to be studied for association with SE was atopic dermatitis5. Since then, evidence has been gathered that SE also has an important role in upper and lower airway disease126. The bacterial allergy, sensitization to SE, is considered to be more important than bacterial infection as a cause and aggravating factor for allergic airway disease7. Recent studies have revealed that SE sensitization is an independent risk factor for asthma and, especially, the severe asthma entity48910. In severe asthmatics, inability to cope with S. aureus colonization in airways by impaired macrophages leads to impairment of mucosal immunity and sensitization to SE11. Asthma represents an inflammatory airway disease with features of mucosal edema and mucus secretion, after allergen stimulation. Airway hyperresponsiveness (AHR), which is measured as increased airway resistance following provocation with stimuli, reflects impairment of mucosal immunity and might be associated with SE sensitization. However, the correlation of SE sensitization with AHR in common asthma patients has never been researched in human clinical studies.
We aimed to evaluate the correlation of SE sensitization with asthma severity, especially AHR, in asthma patients.
We retrospectively enrolled 81 asthma patients whose sIgE to SE data were available and admitted to the Severance Hospital in Korea from March 1, 2013, to February 28, 2015. We retrospectively reviewed the electronic medical records of the enrolled subjects. Asthma was diagnosed by an allergy specialist, based on clinical guidelines, using a bronchodilator test and/or bronchial provocation test12. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (approval number: 4-2013-0397). All enrolled patients provided written informed consent.
The complete blood count test was performed using an automated analyzer to determine blood eosinophil counts. The eosinophil percentages in induced sputum were assessed as follows. The obtained sputum was centrifuged, and the supernatant was collected. Samples were diluted in phosphate-buffered saline and centrifuged at 450 rpm for 6 minutes to prepare cytology slides. After staining the slides with Wright's stain, a differential count was performed using a light microscope, as reported previously13. Forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC) were evaluated by pulmonary function test using commercially available equipment (MS-IOS, Masterlab-IOS; Jaeger, Wurzburg, Germany). AHR was assessed by methacholine challenge test or mannitol challenge test, using a titration protocol as in previous reports. The methacholine challenge started with an inhalation of saline, followed by increasing the concentration of methacholine serially, up to a maximum concentration of 25 mg/mL. We defined positive as the provocative concentration of methacholine required to decrease FEV1 by 20% (PC20), being achieved at less than or equal to 25 mg/mL. The mannitol challenge test ended when a cumulative dose of 635 mg had been inhaled. We defined positive as PD15, the provocative concentration of mannitol required to decrease FEV1 by 15%, less than or equal to 635 mg. All of these tests were conducted before chart review. We retrospectively reviewed the results of the tests.
Allergic rhinitis was clinically diagnosed, based on international guidelines which recommend identifying symptoms, including sneezing, rhinorrhea, nasal obstruction, and itching with specific IgE possession confirmed by skin prick test or ImmunoCAP 250 (Phadia, Uppsala, Sweden) system14. Atopy was defined as positive response to any one of the inhalant allergens (birch, oak, ragweed, mugwort, cat dander, house dust mite, etc.) by ImmunoCAP 250 (Phadia) system, as previously described15.
We measured the levels of sIgE to SE (A/B) in all subjects. First, whole blood was collected in a vacuum tube for serum separation. Serum was separated by centrifugation, and allergen sIgE detection performed using ImmunoCAP 250 (Phadia) system. The system was operated according to the manufacturer's instructions. The sIgE to SE detection range was 0.1 to 100 kU/L.
We assessed correlation between two continuous variables using Pearson's test. The t test was used to compare levels of sIgE to SE according to AHR. We selected, as the parameters, sex and age, which are most commonly among the variables considered, and FEV1 and sputum eosinophil count, which are known to be highly related to asthma severity. We determined the odds ratio (OR) using logistic regression analysis, including univariate and multivariate analysis. Cross-correlation analysis was performed using chi-square tests to analyze the correlation between categorized variables in SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). We considered a p<0.05 to be significant.
We enrolled a total of 81 asthma patients. The mean age of subjects and the standard deviation from the mean was 56.1 years old. The mean value of sIgE to SE and the standard deviation from the mean was 0.260 kU/mL. The FEV1 and FEV1/FVC were assessed by pulmonary function tests and represented a mildly obstructive pattern. The percentage of sputum eosinophils and the standard deviation was elevated beyond the normal range. There was a predominance of females. The prevalence of allergic rhinitis and atopy was 75.3% and 59.5%, respectively. Airway hyperresponsiveness was evaluated in 44 patients, and the results were positive in 45.5%. We defined positive to SE-sIgE as more than 0.10 kU/mL, so that the positivity rate is about 40% (39.5% exactly) (Table 1).
We evaluated the correlation of SE-sIgE with parameters representing asthma severity. The FEV1 (%) and FEV1/FVC (%) were not significantly correlated with the levels of SE-sIgE (p=0.385 and p=0.206, respectively). The serum eosinophils (%) and sputum eosinophil (%) were also not significantly associated with the levels of SE-sIgE (p=0.417 and p=0.815, respectively) (Table 2).
We compared the levels of sIgE to SE according to AHR as assessed by the mannitol challenge test or methacholine challenge test. The sIgE to SE levels in patients with positive AHR (sample size, 20; mean±standard error of the mean, 0.606±0.273 kU/mL) was significantly higher than that in patients with negative AHR (sample size, 24; 0.062±0.015 kU/mL; p=0.034) (Figure 1).
We aimed to identify which parameters could be significant predictive factors for AHR, as assessed by logistic regression. Using univariate analysis, sex (OR, 1.500; 95% confidence interval [CI], 0.400–5.620; p=0.547) and age (OR, 0.714; 95% CI, 0.202–2.528; p=0.602) were not significant predictive factors. The increase in FEV1 (%), indicating better lung function status, was a significant preventive factor (OR, 0.955; 95% CI, 0.914–0.998; p=0.041). The increase in sputum eosinophils (%), sputum eosinophilia, was a significant risk factor (OR, 1.047; 95% CI, 1.009–1.087; p=0.015). Being positive for sensitization to SE (≥0.010 kU/mL) was not statistically significant risk factor for AHR (OR, 2.455; 95% CI, 0.685–8.798; p=0.168). Using multivariate analysis, to control for the other factors, being female was a significant risk factor for AHR (OR, 8.908; 95% CI, 1.009–78.624; p=0.049). The increase in FEV1, which univariate analysis showed to be significant, was not a significant protective factor after adjustment for the other variables (OR, 0.955; 95% CI, 0.906–1.006; p=0.084). The percentage of sputum eosinophils was an independent risk factor for AHR (OR, 1.059; 95% CI, 1.007–1.114; p=0.025). Although positive sensitization to SE was not a significant risk factor when the other factors were not taken into account, it was a significant risk factor for AHR (OR, 7.090; 95% CI, 1.180–42.600; p=0.032) after adjusting for age, sex, FEV1, and sputum eosinophils (Table 3).
When we adjust the cut-off value to 0.014 kU/mL to define 30% of patients as positive to SE sensitization, SE sensitization was a significant risk factor without adjustment (OR, 4.667; 95% CI, 1.037–21.010; p=0.045) and also with adjustment (OR, 14.096; 95% CI, 1.823–108.970; p=0.011).
We assessed whether the prevalence of positive sensitization to SE will be different according to the presence of allergic rhinitis and atopy or not. The positive sensitization rate to SE in patients without allergic rhinitis was 30.0%, and that in patients with allergic rhinitis was 42.6% without statistically significant difference (p=0.431). The positive sensitization rate to SE in non-atopic asthma patients was 40.0%, whereas that in atopic asthma patients was 31.8% (p=0.730) (Table 4).
The novel finding of this study is that SE sensitization might be significantly associated with AHR. Although some researchers have shown the correlation of SE sensitization with asthma diagnosis and severity, effects of SE sensitization on AHR have not been well studied. An allergen-provocation test is a clear-cut way to prove the allergen causes the allergic disease. The direct cause of asthma can be proven by AHR induced by specific allergens. Although the result presented in this study was not a measure of AHR with SE challenge, we revealed that SE sensitization is significantly associated with AHR. This finding, when considered against the significant correlation between SE sensitization and asthma diagnosis and severity described in the literature, more pointedly supports the hypothesis that SE sensitization might lead to asthma.
Actually, study of asthma in the mouse model has already shown that AHR was able to be induced following SE sensitization1617. These studies have explained the mechanism by which SE sensitization induces AHR as follows. SE acts as a superantigen to activate T cells independent of the peptide-binding groove area. SE induces airway inflammation, characterized by increased numbers of lymphocytes and eosinophils and by increased levels of the Th2 cytokine, interleukin 4, together with marked upregulation of tumor necrosis factor α production by macrophages. This inflammatory response may lead to AHR.
Although AHR was assessed in only 44 patients, we found that SE sensitization is significantly associated for AHR. We used a cut-off value of positive sensitization, 0.010 kU/mL, which is widely used in analogous studies81819. When we adjust the cut-off value to 0.014 kU/mL, SE sensitization showed more significant power. SE sensitization might be shown to be a significant independent risk factor for AHR, when a larger number of subjects is examined.
Previous studies revealed that the SE positivity rate is higher in severe asthma than in control and non-severe asthma, but this is still controversial419202122. Rossi and Monesterolo22 reported the significant correlation of SE sensitization with serum eosinophil cationic protein levels, indicating that SE sensitization might be correlated with asthma severity. Kowalski et al.19 found that SE sensitization is significantly correlated with lung function parameters, following adjustment for age. Song et al.10 indicated that SE sensitization may be correlated with sputum eosinophilia. However, the present study could not demonstrate a significant correlation of SE sensitization with asthma parameters, including sputum eosinophilia. We found SE sensitization is significantly correlated with AHR, regard-less of asthma parameters. SE sensitization might be more associated with AHR rather than other asthma parameters, such as lung function and eosinophilia.
We found that SE sensitization was frequently observed in patients with allergic rhinitis (42.6%) compared to patients without (30.0%), but without statistical significance. The failure of this result to reach statistical significance might be due to the small number of subjects studied. Further study with larger sample of allergic rhinitis patients should be performed to evaluate whether SE sensitization is frequently observed in patients with allergic rhinitis rather than patients without.
We also found that the prevalence of SE sensitization was lower in patients with atopic asthma, but without statistical significance. This unresolved association also might yield statistically significant results, were more subjects enrolled. In contrast to atopic asthma, non-atopic asthma is usually associated with elderly age, non-eosinophilic asthma, and severe refractory asthma. Song et al.10 previously described a positive correlation of SE sensitization rate with aging, positive correlation of total IgE with SE sensitization, and inverse correlation of SE sensitization with atopic status9. Our results concerning the inverse correlation of SE sensitization with atopic status are concordant with the previous study described by Song et al.10 Although data are not shown, highly significant correlation between total IgE with SE sensitization (Pearson's coefficient, 0.696; p<0.001) was also concordant with the preious study described by Song et al.10 Further detailed study concerning total IgE and SE sensitization should be conducted to reveal this unresolved issue.
This study was limited to a small patient group. In addition, we could not conduct AHR test in the all enrolled subjects. More significant conclusions concerning asthma severity and AHR will be achieved if we conduct a larger study. Assessment of AHR, when challenged by SE, is likely to reveal the direct association between SE and asthma development. Further basic study should be followed to reveal the mechanisms how the SE sensitization induce AHR and asthma. Although the data are not shown due to the insignificance of results, we found that total IgE (n=7, 713.8±345.9 kU/mL) in AHR positive patients tend to be slightly higher than those (n=5, 182.5±132.3 kU/mL) in AHR negative patients (p=0.244). Further study concerning total IgE with AHR might be interesting. This study has additional limitation concerning severity parameters. The most important severity parameter, medication type and dose used in subjects to control symptoms, was not included in this study. This is due to the deviated distribution to the medium dose inhaled corticosteroid and long-acting β-agonist complex inhaler in almost subjects.
We have, for the first time, revealed that SE sensitization is significantly associated with AHR in common asthma patients, despite the small number of subjects studied. This result implies that SE sensitization might be associated with development of asthma. Further large-scale studies should be conducted to harden these results.
Figures and Tables
Figure 1
Comparison of specific immunoglobulin E (sIgE) to staphylococcal enterotoxin (SE) levels according to airway hyperresponsiveness (AHR) (p=0.034). *p-value<0.05 obtained by t-test.
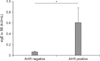
Table 1
Demographics of subjects

Table 2
Correlation of sIgE to SE with parameters associated with asthma

| Parameter | Pearson’s coefficient | p-value |
|---|---|---|
| FEV1 | 0.099 | 0.385 |
| FEV1/FVC | 0.149 | 0.206 |
| Serum eosinophil, % | 0.091 | 0.417 |
| Sputum eosinophil, % | 0.027 | 0.815 |
Table 3
Univariate and multivariate analysis for AHR of data from 44 patients

Table 4
Prevalence of positive to sIgE to SE according to allergic rhinitis and atopy

References
1. Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE 'above atopy'. J Intern Med. 2012; 272:133–143.
2. Pastacaldi C, Lewis P, Howarth P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy. 2011; 66:549–555.
3. Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008; 8:34–38.
4. Bachert C, van Steen K, Zhang N, Holtappels G, Cattaert T, Maus B, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012; 130:376–381.
5. Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB). Allergy. 2000; 55:551–555.
6. Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011; 66:141–148.
7. Bacigaluppi JE, Negroni R, de Severino HM. Bacterial allergy in allergic rhinitis and bronchial asthma. Ann Allergy. 1979; 42:95–98.
8. Tomassen P, Jarvis D, Newson R, Van Ree R, Forsberg B, Howarth P, et al. Staphylococcus aureus enterotoxin-specific IgE is associated with asthma in the general population: a GA(2) LEN study. Allergy. 2013; 68:1289–1297.
9. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014; 44:553–562.
10. Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016; 46:411–421.
11. Liang Z, Zhang Q, Thomas CM, Chana KK, Gibeon D, Barnes PJ, et al. Impaired macrophage phagocytosis of bacteria in severe asthma. Respir Res. 2014; 15:72.
12. Boulet LP, FitzGerald JM, Reddel HK. The revised 2014 GINA strategy report: opportunities for change. Curr Opin Pulm Med. 2015; 21:1–7.
13. Lee JH, Park KH, Park JW, Hong CS. YKL-40 in induced sputum after allergen bronchial provocation in atopic asthma. J Investig Allergol Clin Immunol. 2012; 22:501–507.
14. Bousquet J, Reid J, van Weel C, Baena Cagnani C, Canonica GW, Demoly P, et al. Allergic rhinitis management pocket reference 2008. Allergy. 2008; 63:990–996.
15. Park HJ, Lee JH, Park KH, Kim KR, Han MJ, Choe H, et al. A six-year study on the changes in airborne pollen counts and skin positivity rates in Korea: 2008-2013. Yonsei Med J. 2016; 57:714–720.
16. Huvenne W, Callebaut I, Plantinga M, Vanoirbeek JA, Krysko O, Bullens DM, et al. Staphylococcus aureus enterotoxin B facilitates allergic sensitization in experimental asthma. Clin Exp Allergy. 2010; 40:1079–1090.
17. Herz U, Ruckert R, Wollenhaupt K, Tschernig T, Neuhaus-Steinmetz U, Pabst R, et al. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness: a model for non-allergic asthma. Eur J Immunol. 1999; 29:1021–1031.
18. Bachert C, Gevaert P, Howarth P, Holtappels G, van Cauwen-berge P, Johansson SG. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol. 2003; 111:1131–1132.
19. Kowalski ML, Cieslak M, Perez-Novo CA, Makowska JS, Bachert C. Clinical and immunological determinants of severe/refractory asthma (SRA): association with staphylococcal superantigen-specific IgE antibodies. Allergy. 2011; 66:32–38.
20. Lee JY, Kim HM, Ye YM, Bahn JW, Suh CH, Nahm D, et al. Role of staphylococcal superantigen-specific IgE antibodies in aspirin-intolerant asthma. Allergy Asthma Proc. 2006; 27:341–346.
21. Lee JH, Lin YT, Yang YH, Wang LC, Chiang BL. Increased levels of serum-specific immunoglobulin e to staphylococcal enterotoxin a and B in patients with allergic rhinitis and bronchial asthma. Int Arch Allergy Immunol. 2005; 138:305–311.
22. Rossi RE, Monasterolo G. Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int Arch Allergy Immunol. 2004; 133:261–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download