Abstract
Bronchodilators are the cornerstone of symptomatic chronic obstructive pulmonary disease (COPD) treatment. They are routinely recommended for symptom reduction, with a preference of long-acting over short-acting drugs. Bronchodilators are classified into two classes based on distinct modes of action, i.e., long-acting antimuscarinics (LAMA, once-daily and twice-daily), and long-acting β2-agonists (LABA, once-daily and twice-daily). In contrast to asthma management, evidence supports the efficacy of both classes of long-acting bronchodilators as monotherapy in preventing COPD exacerbations, with greater efficacy of LAMA drugs versus LABAs. Several novel LAMA/LABA fixed dose combination inhalers are currently approved for COPD maintenance treatment. These agents show superior symptom control to monotherapies, and some of these combinations have also demonstrated superior efficacy in exacerbation prevention versus monotherapies, or combinations of inhaled corticosteroids plus LABA. This review summarizes the current data on clinical effectiveness of bronchodilators alone or in combination to prevent exacerbations of COPD.
The term chronic obstructive pulmonary disease (COPD) has been established as an umbrella term to label a clinical syndrome characterized by chronic, poorly reversible airflow obstruction, airway inflammation in the presence of chronic bronchitis and/or pulmonary emphysema1. It is, however, increasingly recognized, that distinct COPD phenotypes exist, and these may be prone to a more personalized, "targetted" management approach2. In this regard, a "frequent exacerbator" phenotype has been identified, and exacerbation risk is now used to classify COPD patients according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy paper (GOLD groups C and D, subjects with a history of 2 moderate, or one severe hospitalized exacerbation in the past years)1.
Exacerbations are considered key events in the clinical course of COPD, and the prevention of exacerbations is highlighted as a pivotal therapeutic goal and relevant outcome measure by current treatment strategies or guidelines. While these events are somehow associated with the severity of COPD, the distribution of exacerbations in COPD is not uniform, with seasonal or temporal clustering34, in particular in a subset of COPD patients at high risk for exacerbations, where the individual history of prior exacerbations is the strongest single predictor of future events5.
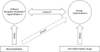
Current strategies recommend long-acting bronchodilators as first line maintenance therapy for symptomatic COPD1. Long-acting bronchodilators produce consistent improvements in lung function and patient-centred outcomes, including airflow (forced expiratory volume in 1 second), hyperinflation (inspiratory capacity or functional residual capacity), symptomatic control of dyspnea, health-related quality of life, exercise capacity, prevention of exacerbations, and, potentially, mortality in subsets of patients167. To achieve bronchodilation, two classes of drugs are currently available, namely long-acting muscarinic antagonists (LAMA), and long-acting β2-agonists (LABA). Both classes of agents have been demonstrated to be clinically effective, with acceptable safety profiles7. Importantly, the combined use of two bronchodilators with different mechanism of action is considered an effective strategy to optimize bronchodilation in COPD, and there is now ample evidence for the improved clinical efficacy of fixed combinations of long-acting bronchodilators versus monotherapies or inhaled corticosteroid (ICS)/LABA combinations, in particular with regards to functional and symptomatic outcomes891011. Importantly, in COPD, bronchodilators can also effectively prevent exacerbations of the disease, as single agents or in combinations. The potential mechanisms, by which bronchodilators may prevent exacerbations have been under discussion. These may include direct effects on airflow, reduced hyperinflation, thus leading to improved respiratory mechanics and increased thresholds for development of symptoms, but also indirect mechanisms (improved secretion clearance through better airway patency) and anti-inflammatory properties of bronchodilators (reduced sputum production, cytokine release) have been proposed (Figure 1). An in-depth review on this topic was published by Wedzicha et al.12.
This review will discuss the available evidence of the clinical effectiveness of long-acting bronchodilators to prevent COPD exacerbations. As ICS have also demonstrated efficacy in decreasing exacerbations13, an important question that is now being addressed in many clinical trials is which combinations of these agents have the greatest benefit for decreasing exacerbations.
Available LABAs for the maintenance treatment of COPD include twice-daily drugs with approx. Twelve hours duration of action (formoterol [FOR] and salmeterol [SAL]), and once-daily drugs with 24 hour bronchodilator effect (indacaterol [IND] and olodaterol [OLO]). Recent meta-analyses on the preventive effect of the older drugs SAL and FOR confirm some clinical benefits of these drugs on exacerbations, although results from single studies are conflicting714. It must be considered that most of the studies with data on exacerbations with twice-daily LABAs were not powered to show an effect on exacerbation prevention as primary study endpoint. The largest body of evidence has been generated with SAL, often in the context of the pivotal trials that led to approval of the ICS/LABA combination fluticasone/salmeterol (FLU/SAL), where SAL monotherapy was used as active comparator. In the Towards a Revolution in COPD Healthcare (TORCH) long-term trial, SAL twice-daily over 3 years in 6,112 COPD patients led to a significant reduction of the overall annualized exacerbation rate, the number of exacerbations requiring systemic corticosteroids and/or hospitalization versus placebo15.
In contrast, during a one year trial in 812 patients with budesonide/formoterol with FOR monotherapy as control arm, FOR 12 µg twice-daily did not reduce moderate or severe exacerbations versus placebo16.
IND, a once-daily LABA approved at doses of 75 (United States), 150 or 300 µg (Europe and Asia) was shown to reduce moderate-to-severe exacerbations in studies over at least 6 months duration versus placebo, in a post-hoc analysis of randomized trials in 2,716 moderate-to-severe COPD patients. Indacaterol at doses of 150 and 300 µg significantly reduced the COPD exacerbation rates compared with placebo by 31% and 29%, respectively (both p=0.002), and also significantly prolonged the time to first moderate-to-severe exacerbation versus placebo17.
OLO is another once-daily LABA licensed for the maintenance treatment of COPD (approved dose 5 µg, once-daily). In phase III long-term trials over 48 weeks, clinical benefits in terms of lung function and symptomatic improvements comparable to other available LABAs were observed; however, exacerbation were collected only as safety endpoints, with no conclusive effect of OLO versus placebo or comparators1819.
Finally, the once-daily LABA vilanterol (VI) is used as a partnering agent for the ICS/LABA combination fluticasone furoate/vilanterol (FF/VI) and the LAMA/LABA combination umeclidinium/vilanterol (UMEC/VI). In the large-scale "Study to Understand Mortality and Morbidity" (SUMMIT) randomized trial over a median study period of 1.8 years in 16,590 COPD patients20, VI monotherapy reduced the annual rate of moderate/severe exacerbations versus placebo (0.31 vs. 0.35, p=0.017). However, VI is not licensed as a monotherapy for the maintenance therapy of COPD.
LAMAs are considered a first-line option for the long-term maintenance treatment of COPD. Of these, the richest body of evidence exists for tiotropium (TIO) once-daily (18 µg delivered via HandiHaler or 5 µg via Respimat Soft Mist Inhaler), with experience from clinical trials and everyday practice of >10 years. The preventive effect of TIO on exacerbations has been repeatedly and consistently demonstrated in well-controlled long-term trials. In particular, two studies with >1,000 patients were specifically designed to evaluate the effect of TIO versus placebo on moderate or severe, hospitalized exacerbations over 6 and 12 months treatment duration2122. Both studies showed consistent reductions in the total number of exacerbations, the proportion of subjects experiencing at least one exacerbation, and prolongation of the time-to-first exacerbation. In the study by Niewoehner et al.22, there was also a trend towards reduction of hospitalized exacerbations with tiotropium versus placebo. Finally, in the long-term UPLIFT trial, TIO was superior to the control arm in a number of exacerbation outcomes23. Exacerbation prevention with tiotropium delivered by the Respimat was also shown to be non-inferior to the HandiHaler device in the large-scale TIOSPIR trial24.
In head-to-head comparator trials, TIO once-daily was superior to SAL twice-daily in all exacerbation-related outcomes25. The superiority of the LAMA TIO was further confirmed in a head-to-head trial versus the once-daily LABA IND, where TIO significantly prolonged the time to first moderate-to-severe exacerbation versus LABA26. Finally, in another head-to-head trial of TIO versus ICS/LABA, there was no difference between treatments in reducing the overall annual rate of moderate-to-severe exacerbations27. Altogether, these results led to the recommendation of using the LAMA TIO as a fist-line treatment also in subjects with a high risk for exacerbations (GOLD 2016 groups C and D).
More recently, additional LAMA drugs have been introduced into the COPD management options, including once-daily glycopyrronium (licensed as twice-daily drug in the United States), umeclidinium, and the twice-daily drug aclidinium.
In a 1-year phase III trial28, glycopyrronium 50 µg once-daily reduced the risk of a moderate-to-severe exacerbation in moderate-to-severe COPD patients by 34% versus placebo, an effect comparable in magnitude to the effect of tiotropium in the control arm (open label, 39% reduction vs. placebo).
For umeclidinium (62.5 µg once-daily), no data on exacerbation prevention have been reported this far. Effects on other clinical outcomes in studies over 3 to 6 months duration suggest comparable efficacy to other LAMAs, though29.
Exacerbations (diary or healthcare resource utilization defined) were evaluated as secondary outcome parameter in the 24-week randomized, placebo-controlled ATTAIN trial with twice-daily aclidinium (400 µg twice-daily)30. In this study, a 33% reduction in the overall exacerbation rate versus placebo was observed with aclidinium. Longer trials with exacerbations as primary endpoint, however, have not been performed. A recent systematic review performed by the German Institute for Quality and Efficiency in Healthcare (IQWIG) as part of the mandatory national value assessment procedure for novel drugs, using data from the aclidinium/formoterol fixed combination pivotal phase III trials, suggests there is some incremental benefit of aclidinium over formoterol twice-daily in preventing severe exacerbations31.
Altogether, the current evidence suggests, that LAMAs, in particular TIO, represent the most effective monotherapy to prevent exacerbations of COPD. Hence, the first-line recommendation of this class of bronchodilator as preferred option over LABAs for maintenance therapy in patients with high risk of exacerbations appears clearly justified.
Unlike in asthma, there is no recommendation for ICS monotherapy in COPD, based on their limited efficacy on relevant outcomes in COPD. ICS, however, may improve clinical effects observed with bronchodilator monotherapy alone, in particular when combined with LABAs. The primary domain of ICS/LABA in COPD is the prevention of exacerbations in high-risk patients with at least severe airflow limitation. Available ICS/LABA combinations include twice-daily FLU/SAL, budesonide/formoterol (BUD/FOR), beclomethasone/formoterol, and once-daily FF/VI. ICS/LABA combinations consistently reduce exacerbation rates in high-risk patients versus placebo, although less consistently against monotherapies3233. For example, in the TORCH trial, FLU/SAL reduced the rate of moderate/severe exacerbations versus placebo by 25%, versus SAL by 12%, while the rate of severe hospitalized exacerbations was not different from SAL monotherapy15. In the TRISTAN study, moderate/severe exacerbation rates, in contrast, were similar between ICS/LABA and LABA monotherapy34. Similar results have been seen with BUD/FOR versus LABA component alone. A more recent meta-analysis estimated the exacerbation reduction of ICS/LABA versus LABA alone by 17%, without demonstrable effect on hospitalized exacerbations32. As described earlier, an ICS/LABA combination was not superior to a LAMA alone in preventing exacerbations in the INSPIRE study27.
Since the year 2013, a once-daily ICS/LABA combination, FF/VI, has been approved for COPD. In two pivotal phase III studies, the combination of FF/VI reduced exacerbation rates versus VI alone in one study, while the other study did not show any difference35. More recently, in the SUMMIT study20 a numerical reduction of exacerbations with FF/VI versus VI alone was seen (0.25 vs. 0.31, no formal statistical comparison was reported), but this study included COPD patients with a low exacerbation risk (GOLD B). In line with other studies, the occurrence of pneumonia was increased with ICS/LABA combination therapy versus LABA alone35.
It has been known for decades, that in COPD a combination of bronchodilator drugs with different mode of action provides better lung function improvements and clinical outcomes than one drug alone36. The first study specifically designed to evaluate the impact of combined bronchodilator therapy on exacerbations was published in 200737. In this study, an accepted definition for an exacerbation was used, the study duration was appropiate (52 weeks), and exacerbations were the primary endpoint. Over the study period of 1 year, no difference in the incidence of exacerbation was observed for tiotropium vs tiotropium plus SAL or SAL/FLU (62.8% of patients vs. 64.8% and 60.0%, respectively). In addition, the time to first exacerbation was not different between treatment arms. However, the study was limited by a high dropout rate, therefore further studies evaluating the benefits of dual bronchodilator therapy versus single agents in carefully selected patient groups were required.
With the advent of long-acting bronchodilator drugs, several novel LAMA/LABA fixed combinations have been approved for COPD maintenance treatment (Table 1). These include once-daily options glycopyrronium/indacaterol (GP/IND), tiotropium/olodaterol (TIO/OLO), UMEC/VI, and finally, twice-daily aclidinium/formoterol (ACL/FOR). Consistently, these combinations provide superior lung function improvements versus bronchodilator monotherapies and ICS/LABA combinations, while also improving patient-related outcomes like dyspnea or quality of life in most controlled trials89101138. Given the excellent efficacy of LAMA monotherapy in preventing exacerbations, it was tempting to speculate on whether these novel dual combinations with superior, at times so far unseen, bronchodilator efficacy would also lead to further improvements in exacerbation prevention, in particular as—on average—exacerbation frequency increases with airflow obstruction, and a reasonable correlation of the magnitude of hyperinflation with exacerbation occurrence have been observed. Indeed, this question has now been addressed in a few controlled clinical trials with LAMA/LABA combination therapy versus active controls, using exacerbations as key outcomes.
Firstly, the SPARK Study evaluated the effect of 1-year treatment with once-daily GP/IND (50/110 µg) on the annual rate of moderate/severe exacerbations in 2,224 severe to very severe COPD patients with a history of at least one moderate exacerbation in the years prior to study entry (GOLD groups C and D), using LAMA monotherapy (GP 50 µg once-daily, open-label TIO 18 µg once-daily) as active control39. For all exacerbations (mild, moderate, and severe), GP/IND reduced the annual exacerbation rate by 15% and 14% versus GP and TIO, respectively (p=0.001 and p<0.01). Regarding the primary endpoint (moderate/severe exacerbations), GP/IND significantly reduced these events versus GP alone by 12% (p<0.05), while a nonsignificant reduction by 10% was seen versus open label TIO (p=0.09). IND/GP also improved health-related quality of life (St. George's Respiratory Questionnaire) significantly versus both controls.
In the pivotal phase III TONADO replicate 1-year trials comparing TIO/OLO fixed combination (5/5 µg once-daily via Respimat) versus single components, the occurrence of exacerbations was lowest in the TIO/OLO combination group as compared to the monotherapy comparators8. However, numerical trends were observed only, and TONADO trials were no dedicated exacerbations trials. Nonetheless, given these encouraging results, the comparative efficacy of TIO/OLO over TIO monotherapy is currently evaluated in a large, long-term study using various exacerbation outcomes as primary endpoints (DYNAGITO; Clinicaltrial.gov identifier: NCT02296138). Hopefully, this study will shed more light on the potential superior efficacy of dual bronchodilation to prevent exacerbations versus the current "gold standard," TIO.
Recently, results from a head-to-head comparator trial of GP/IND once-daily versus a twice-daily ICS/LABA combination (FLU/SAL) were reported (FLAME Study)40. The study aimed to demonstrate non-inferiority of the dual LAMA/LABA combination versus ICS/LABA in terms of the total annual exacerbation rate. While non-inferiority was demonstrated, the secondary analyses also proved superiority of the LAMA/LABA combination over ICS in reducing the rate of all (mild, moderate, and severe) and moderate/severe exacerbations. This was supported by superiority of several additional exacerbation endpoints (time-to-first mild, moderate, and severe exacerbation), as well as improvements in lung function and health status. The FLAME study therefore for the first time demonstrated superiority of a "pure" bronchodilator approach versus a regime containing an anti-inflammatory component to prevent exacerbations in high-risk subjects with COPD. Strikingly, the superior effect of the dual bronchodilation approach was independent of the baseline level of blood eosinophils, a biomarker that has recently been suggested to suit as a guidance to identify COPD patients with responsiveness to the exacerbation prevention effect of ICS41.
For both UMEC/VI and ACL/FOR, no dedicated exacerbations studies have been performed. For ACL/FOR, some subgroup analyses suggest a benefit of ACL/FOR versus FOR monotherapy in preventing exacerbations (IQWIG)42, however these effects require confirmation in longer-term studies.
Bronchodilators are the cornerstone COPD management. They are recommended on a regular basis to prevent or reduce symptoms, improve health status and exercise tolerance. Importantly, long-acting bronchodilators also prevent the occurrence of exacerbations, with superior efficacy of LAMA over LABA in head-to-head trials. With two classes of bronchodilators with distinct, yet complementary mode of action available (LAMA and LABA), either class of drug can be given alone or in combination, the latter is now possible in form of fixed drug combinations. Currently, the role of LAMA/LABA dual bronchodilator drugs is seen as alternative options in more severly symptomatic patients, not necessarily with a background or risk of exacerbations (Table 2). However, given the superior clinical efficacy in improving lung function and symptoms versus monotherapies, this view will likely change in the near future. Importantly, recent large scale trials indicate the superiority of a pure dual bronchodilator approach over LAMA monotherapy and ICS/LABA combinations also in high-risk exacerbators (COPD GOLD groups C and D), challenging our current clinical practice to optimally prevent exacerbations in these patients. in the future, development of "triple therapies" (ICS/LAMA/LABA) will help to clarify a potential role of ICS when added to "optimal" bronchodilator background.
Figures and Tables
Table 1
Overview of dual LAMA/LABA fixed combination currently approved

Table 2
GOLD 2016: pharmacologic therapy for stable COPD

Adopted from Global Initiative for Chronic Obstructive Lung Disease (2016)1, http://www.goldcopd.org.
GOLD: Global Initiative for Chronic Obstructive Lung Disease; COPD: chronic obstructive pulmonary disease; SAMA: short-acting antimuscarinic; SABA: short-acting beta-agonist; LAMA: long-acting antimuscarinics; LABA: long-acting β2-agonists; PDE-4: phosphodiesterase 4.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2016 update [Internet]. Global Initiative for Chronic Obstructive Lung Disease;2016. cited 2016 Jul 9. Available from: http://www.goldcopd.org.
2. Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010; 182:598–604.
3. Rabe KF, Fabbri LM, Vogelmeier C, Kogler H, Schmidt H, Beeh KM, et al. Seasonal distribution of COPD exacerbations in the Prevention of Exacerbations with Tiotropium in COPD trial. Chest. 2013; 143:711–719.
4. Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009; 179:369–374.
5. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.
6. Vogelmeier C, Buhl R, Criee CP, Gillissen A, Kardos P, Kohler D, et al. Guidelines for the diagnosis and therapy of COPD issued by Deutsche Atemwegsliga and Deutsche Gesellschaft fur Pneumologie und Beatmungsmedizin. Pneumologie. 2007; 61:e1–e40.
7. Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MM. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006; (3):CD001104.
8. Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, Ferguson G, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015; 45:969–979.
9. Beeh KM, Derom E, Echave-Sustaeta J, Gronke L, Hamilton A, Zhai D, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat((R)) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler((R)) (ENERGITO((R)) study). Int J Chron Obstruct Pulmon Dis. 2016; 11:193–205.
10. Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013; 42:1484–1494.
11. Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013; 1:51–60.
12. Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012; 40:1545–1554.
13. Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest. 2010; 137:318–325.
14. Wang J, Nie B, Xiong W, Xu Y. Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis. J Clin Pharm Ther. 2012; 37:204–211.
15. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.
16. Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003; 21:74–81.
17. Wedzicha JA, Buhl R, Lawrence D, Young D. Monotherapy with indacaterol once daily reduces the rate of exacerbations in patients with moderate-to-severe COPD: Post-hoc pooled analysis of 6 months data from three large phase III trials. Respir Med. 2015; 109:105–111.
18. Koch A, Pizzichini E, Hamilton A, Hart L, Korducki L, De Salvo MC, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat(R) versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014; 9:697–714.
19. Ferguson GT, Feldman GJ, Hofbauer P, Hamilton A, Allen L, Korducki L, et al. Efficacy and safety of olodaterol once daily delivered via Respimat(R) in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014; 9:629–645.
20. Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016; 387:1817–1826.
21. Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006; 27:547–555.
22. Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA Jr, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143:317–326.
23. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554.
24. Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013; 369:1491–1501.
25. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011; 364:1093–1103.
26. Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013; 1:524–533.
27. Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008; 177:19–26.
28. Kerwin E, Hebert J, Gallagher N, Martin C, Overend T, Alagappan VK, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012; 40:1106–1114.
29. Feldman G, Maltais F, Khindri S, Vahdati-Bolouri M, Church A, Fahy WA, et al. A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 mug compared with tiotropium 18 mug in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016; 11:719–730.
30. Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012; 40:830–836.
31. Institut für Qualität und Wirtschaflichkeit im Gesundheitswesen. Aclidiniumbromid: Nutzenbewertung nach §35 SGB V. A15-45 [Internet]. Köln: IQWIG;2016. cited 2016 Jul 9. Available from: http://www.g-ba.de/downloads/92-975-1206/2016-01-15_Nutzenbewertung-IQWiG_Aclidiniumbromid.pdf.
32. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; (9):CD006829.
33. Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014; 9:469–479.
34. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003; 361:449–456.
35. Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013; 1:210–223.
36. Petty TL. The combination of ipratropium and albuterol is more effective than either agent alone. Chest. 1995; 107:5 Suppl. 183S–186S.
37. Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007; 146:545–555.
38. Mahler DA, Decramer M, D'Urzo A, Worth H, White T, Alagappan VK, et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014; 43:1599–1609.
39. Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013; 1:199–209.
40. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016; 374:2222–2234.
41. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015; 3:435–442.
42. Institut für Qualität und Wirtschaflichkeit im Gesundheitswesen. Aclidiniumbromid/Formoterol. Nutzenbewertung nach §35 SGB V. A15-06 [Internet]. Köln: IQWIG;2015. cited 2016 Jul 9. Available from: http://www.g-ba.de/downloads/92-975-767/2015-05-04_Nutzenbewertung-IQWiG_A15-06_Aclidiniumbromid-Formoterol.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download