Abstract
Chronic obstructive pulmonary disease (COPD) encompasses several clinical syndromes, most notably emphysema and chronic bronchitis. Most of the current treatments fail to attenuate severity and progression of the disease, thereby requiring better mechanistic understandings of pathogenesis to develop disease-modifying therapeutics. A number of theories on COPD pathogenesis have been promulgated wherein an increase in protease burden from chronic inflammation, exaggerated production of reactive oxygen species and the resulting oxidant injury, or superfluous cell death responses caused by enhanced cellular injury/damage were proposed as the culprit. These hypotheses are not mutually exclusive and together likely represent the multifaceted biological processes involved in COPD pathogenesis. Recent studies demonstrate that mitochondria are involved in innate immune signaling that plays important roles in cigarette smoke-induced inflammasome activation, pulmonary inflammation and tissue remodeling responses. These responses are reviewed herein and synthesized into a view of COPD pathogenesis whereby mitochondria play a central role.
Chronic obstructive pulmonary disease (COPD) encompasses several clinical syndromes, most notably emphysema and chronic bronchitis12. It is a major unmet medical burden worldwide and, in western society, is strongly associated with cigarette smoke (CS) exposure. Estimates from World Health Organization (WHO)'s Global Burden of Disease and Risk Factors project show that in 2001, COPD was the fifth leading cause of death in high-income countries, accounting for 3%–8% of total deaths, and it was the sixth leading cause of death in nations of low and middle income, accounting for 4%–9% of total deaths3. In this same report, COPD was also estimated to be the seventh and 10th leading cause of disability-adjusted life years in countries of high income and in those of low or middle income, respectively3. COPD tissues are characterized by chronic inflammation, mucus metaplasia, alveolar destruction, and structural cell apoptosis2. It is important to point out; however, that the underlying mechanisms of the pathogenesis of COPD have not yet been adequately elucidated, hindering the successful development of disease-modifying therapeutics.
Recently, mitochondria/mitochondrial dysfunction has been highlighted in a variety of disorders and human health4. Mitochondria take important parts not only in cellular respiration but also in other fundamental cellular functions including metabolism, innate and adaptive immune signaling, calcium homeostasis, senescence, and cell death. Accordingly, recent studies have revealed unprecedented roles of mitochondrial molecules which play in the context of COPD pathogenesis. These recent advancements on mitochondrial biology have enabled us to envisage that COPD pathogenesis could be understood better if it is focused on from the mitochondrial perspective. In this review, current state-of-art understanding of mitochondrial biology and mitochondrial molecules in the context of COPD pathogenesis is discussed.
A number of major theories of COPD pathogenesis have been promulgated. Initially, since the 1960s, the protease/anti-protease hypothesis dominated thinking in this area. And, the concept has built up that the increase in protease burden is thought to derive from inflammatory cells (hence the "Inflammation Hypothesis" of pathogenesis). In addition, the "Apoptosis Hypothesis," which proposes that apoptosis/cell death response caused by cellular injury/damage is a primary event in the pathogenesis of pulmonary emphysema, has been highlighted in the field of COPD research. And for a long time, exaggerated production of reactive oxygen species (ROS) and resulting oxidant injury have been postulated to be a major event in the pathogenesis of COPD (Oxidant Injury Hypothesis). These concepts and others that are widely discussed to explain COPD pathogenesis are briefly summarized below.
In 1964, researchers reported that a deficiency of α1-antitrypsin was associated with emphysema5. A few years later, neutrophil elastase was reported to be the target of α1-antitrypsin. These findings, together with the observation of increased numbers of neutrophils and macrophages in the lungs of smokers, link various proteases from these inflammatory cells as the primary effectors of lung destruction in COPD678. In this concept, the normal lung is believed to be protected by an antiprotease "shield" that negates the function of proteolytic enzymes that are released into the airway or parenchyma, and emphysema is believed to be caused by an increase in proteases and or a reduction in antiproteases.
As noted in the current definition, COPD is characterized by airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases9. Inflammation with infiltrating macrophages, neutrophils, lymphocytes and occasionally eosinophils, is seen throughout the bronchial tree and parenchyma of lungs from patients with COPD. In addition, airway inflammation is believed to start at an early stage, many years prior to the onset of clinical symptoms, in patients with COPD10. It is important to note that substantial heterogeneity is observed in lungs from patients with COPD. Although exaggerated type 1 inflammation plays an important role in the pathogenesis of emphysema1112, recent reports have also highlighted type 2- and type 17-cytokine productions in this disorder (reviewed in Brusselle et al.13). In this regard, it is interesting that a study demonstrated that a pleiotrophic proinflammatory cytokine, interleukin (IL)-18, can simultaneously induce the signature cytokines associated with type 1, type 2 and type 17 responses and that each of these plays a specific role in the pathogenesis of the IL-18 effector repertoire14. This study demonstrated also that IL-18 mediated signaling induces chronic inflammation, emphysema, mucus metaplasia, airway fibrosis and vascular remodeling with intimal hyperplasia, which mimic, in many ways, the pathologies in human COPD14. As known, the increase of IL-1β and IL-18, target cytokines of inflammasome activation, in COPD patients has been demonstrated in multiple studies including ours1516. Overall, the inflammation in COPD lung tissues is believed to be causally related to emphysema development and other pathologic alterations in the lungs that worsen with disease progression.
An increased burden of oxidative stress is an important feature of the pathogenesis of COPD and, for a long time, oxidative stress resulting from an imbalance between oxidants and antioxidants has been proposed as the basis for COPD. Patients with COPD experience an increased burden of oxidative stress due to the combined effects of excess ROS and reactive nitrogen species generation, antioxidant depletion and reduced antioxidant enzyme activity. In addition, CS increases the burden of oxidants in the respiratory tract, either directly included in the CS or generated by inflammatory cells, depleting antioxidant defenses and injuring lung cells17. Furthermore, the increase of oxidative stress is compounded by alterations in the antioxidant defenses in patients with COPD8. Current knowledge on this theme has been extensively reviewed in other publications.
The lung is unique in that alveolar cells are constantly exposed to external physical and chemical stresses. As physiologic responses upon these cellular injury/damage, apoptotic cell removal and cellular replacement are believed to be highly regulated to maintain physiologic homeostasis of the entire alveolar unit18. Therefore, overinduction of apoptosis and/or blockade of cellular replenishment would be expected to disrupt alveolar septal homeostasis and the "Apoptosis Hy-pothesis" highlights that pulmonary structural cell apoptosis/death response due to ongoing cellular injury/damage as a critical element in the COPD pathogenesis (reviewed in Tuder et al.18 and Yoshida and Tuder19). Indeed, alveolar septal cell apoptosis was confirmed in lungs from patients with COPD20. Interestingly, this study also suggested that CS-induced injury might activate mitochondrial intrinsic pathway of apoptosis, which was demonstrated by immunohistochemistry in COPD lungs20.
Aging is thought to be a degenerative process caused by accumulated damage that leads to cellular dysfunction, tissue failure, and death2122. Similar to other organ systems, biological aging of the pulmonary system is associated with structural changes leading to a progressive decline in function, and the term "aging lung" is usually applied to designate the organ2324. Interestingly, the hallmarks of ageing, which include telomere shortening, genomic instability, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, deregulated nutrient-sensing and stem cell exhaustion, are all observed in COPD lungs25. In this sense, studies have long been sought to understand COPD pathogenesis in the perspective of accelerated aging.
In this theory, a series of innate and adaptive immunologic responses are proposed as major events which contribute to COPD pathogenesis; CS activates innate immune cells such as epithelial cells and macrophages by triggering pattern recognition receptors (PRRs) or via the release of danger-associated molecular patterns (DAMPs) from stressed or dying cells; the signals from various innate immune stimulation lead to the activation of dendritic cells and subsequently, induce adaptive immune responses encompassing T helper (Th1 and Th17) CD4+ T cells, CD8+ cytotoxicity, and B-cell responses; in addition, viral and bacterial infections contribute to not only the pathogenesis of acute exacerbations of COPD but also the amplification and perpetuation of chronic inflammation in stable COPD via dysregulation of a variety of innate and adaptive immune signaling pathways. Furthermore, the potential role of autoimmunity including autoantibodies in the development of COPD has been suggested also. These recent understandings have been comprehensively reviewed in other publications121326.
Historically, the major role of mitochondria has been thought to be the powerhouse of the cell because these organelles catalyze oxidation of metabolites to drive ATP production via the process of oxidative phosphorylation (OXPHOS)27. OXPHOS involves transfer of high-energy electrons derived from NADH and FADH2 generated by the TCA cycle through the electron transfer chain (ETC) complexes that ultimately donate electrons to molecular oxygen. During this process, protons are pumped across the inner mitochondrial membrane resulting in a proton-motive force that is utilized to produce ATP by the F0F1 ATP synthase27. While this is clearly a major function of mitochondria, studies over the past two or three decades have revealed additional critical functions of mitochondria, including regulation of cell proliferation, death and differentiation, redox and calcium homeostasis and others. One exciting new area is how mitochondria dictate immune responses via regulation of a broad range of innate immune pathways and the determination of immune cell phenotype and their functions2829. Hence, considering that mitochondrial function and behavior are fundamental to the physiology of cellular and organismal health, it is not surprising "mitochondrial dysfunction" has been implicated in a wide range of diseases that encompass all aspects of medicine (reviewed in Nunnari and Suomalainen4). For example, the pathogenic role of mitochondrial dysfunction has been increasingly identified in a large number of human diseases including diabetes, Alzheimer's, Parkinson's, cardiac dysfunction, tumorigenesis, pulmonary disorders as well as aging3031. Below we describe the role of mitochondria in COPD pathogenesis based on recent studies in this area.
Inflammation is a fundamental response of the innate immune system to noxious stimuli. The concept of inflamma-somes was introduced nearly a decade ago, and since then, they have been increasingly recognized as central players in innate immune and inflammatory responses in settings of infection and sterile inflammation32. Active caspase-1, formed by the activation of inflammasomes, is used for the activation of pro-inflammatory cytokines IL-1β and IL-18, and release of these cytokines results in the recruitment of effector cell populations important in the immune response and tissue repair. During infection or injury, inflammasomes are directly or indirectly activated by a wide array of pathogen-associated molecular patterns (PAMPs) or DAMPs and, under normal circumstances, activation of inflammasomes culminates in the resolution of infection or inflammation and contributes to homeostatic processes. However, in a pathologic context, perpetuation of inflammasome activation can lead to a variety of chronic inflammatory conditions such as metabolic disorders, tumorigenesis and autoimmunity323334. The roles of mitochondria on inflammasome regulation have been explored in multiple circumstances and we have recently addressed this in the context of COPD pathogenesis35.
Mitochondria are an important source of ROS within most mammalian cells36. Mitochondrial ROS have a crucial roles in a variety of physiological and pathological circumstances3037. In addition, mitochondria are themselves prone to oxidative damage, which results in further perturbation of mitochondrial function. While ROS have long been appreciated for their damage-promoting, detrimental effects, there is now a greater understanding of their roles as signaling molecule and mitochondrial ROS-mediated signaling pathways are involved in various basal and adaptive physiological responses3839. It should be noted that our understanding of mitochondrial redox signaling pathways is still in its infancy, but unraveling these holds therapeutic potential for many diseases including COPD.
Mitochondria represent a physical point of convergence for many cell death-inducing signals in mammalian cells4041. Mitochondria not only adjudicate the decision of whether or not to commit to cell death, but also release pro-apoptotic proteins that are involved in widespread proteolysis, nucleolysis, and cell engulfment. Interactions among BCL-2 family proteins at the mitochondrial outer membrane control the release of these toxic factors and the commitment to apoptosis40. Furthermore, several perturbations of intracellular homeostasis are specifically detected by mitochondria, thereby involving them in the initiation of cell death. In summary, mitochondria regulate the transition between cell death initiation and execution in a vast majority of settings, and thus, mitochondria stand out as key controllers of cell death subroutines initiated at most subcellular locations41.
Mitochondria are centrally positioned signaling hubs that regulate of innate and adaptive immunity2829. The discovery of mitochondrial antiviral signaling (MAVS) protein, which was the first mitochondria-localized protein implicated in the antiviral innate immune response, was a breakthrough in establishing the role for mitochondria in innate immunity42. Interestingly, recent studies have suggested that the MAVS-mediated signaling might play an important role in the pathogenesis of COPD4344 (this topic is discussed further in next section). Furthermore, multiple studies have revealed that mitochondrial signaling controls adaptive immunity including CD4+ T cell differentiation, CD8+ memory T cell formation and others via various mitochondrial metabolic pathways (reviewed in Weinberg et al.29). For example, mitochondrial aerobic glycolysis was found to be required for effective T cell activation through generation of mitochondrial ROS, which are necessary for optimal activity of nuclear factor of activated T cells and proximal T-cell receptor–mediated signaling4546. It is important to note that the role of mitochondria which take part in COPD immunology has not been adequately elucidated. Future studies to define underlying mechanisms by which mitochondria and mitochondrial signaling molecules function in the immunologic aspect of COPD pathogenesis will be beneficial not only for our enhanced understanding on this area but also for the development of potential disease-modifying therapeutics.
Multiple studies have begun to define mitochondrial function or molecules that contribute to the pathogenesis of various pulmonary disorders, including COPD. For example, nucleotide binding domain and leucine-rich-repeat-containing protein X1 (NLRX1) and MAVS, novel mitochondrial molecules initially known to regulate cytoplasmic nucleic acid-mediated innate immune pathway, have recently been implicated in the pathogenesis of COPD44. More intriguingly, most of characteristic features involved in COPD pathogenesis, which include enhanced inflammasome activation and intraalveolar inflammation, exaggerated proteases and cytokine responses, dysregulation of ROS production as well as enhanced alveolar cell apoptosis/cell death, were attributed to CS-induced dysregulation of NLRX1/MAVS-mediated signaling44. These observations further support the notion that CS-induced mitochondrial dysfunction or dysregulation of mitochondrial molecules could be key features to drive development of COPD.
NLRX1 is the member of the NLR family of PRRs that has a unique N-terminal domain, which accounts for the letter "X" in its acronym. It contains a mitochondrial targeting sequence, and biochemical analyses demonstrated that NLRX1 localizes to mitochondria47. Strikingly, NLRX1 represents the first, and so far only, PRR family member that targets this cellular location, and hence a likely link between mitochondrial functions and innate immunity48. Initially, it was identified to function as a negative regulator of RIG-I signaling by targeting the RIG-I downstream MAVS4950. As noted, a recent study has identified NLRX1 as an important player in the pathogenesis of COPD44, whereby it was significantly suppressed in mice exposed to CS, and CS-induced activation of inflammasome(s), pulmonary inflammation and emphysematous destruction were exaggerated in the absence of NLRX144. The importance of NLXR1 was also evident from clinical studies. In three independent human COPD cohorts, the expression of NLRX1 was suppressed in lungs from patients with COPD and this suppression showed strong correlation with the degree of airflow limitation, a hallmark of COPD44. Overall, these studies highlight NLRX1 as a critical inhibitor in the pathogenesis of COPD (Figure 1) and other studies suggest additional ways that it is involved in mitochondrial regulation, including interactions with mitochondrial Tu translation elongation factor to promote autophagy51, with UQCRC2, a subunit of ETC complex III to regulates ROS generation47 and tumor suppressor activity52 that could be relevant to the disease.
As noted above, MAVS was the first mitochondria-localized protein causally linked to antiviral innate immune responses42. It is now recognized that in addition to induction of antiviral and inflammatory responses, MAVS interacts with many molecules that have roles in antiviral responses, inflammation, apoptosis, mitochondrial dynamics, autophagy and proteasome degradation. In this regard, it is interesting that MAVS appeared to have a critical role in the exaggerated production of IL-18 production as well as pulmonary inflammatory and remodeling responses that are observed after CS and respiratory virus or viral PAMP co-exposures in a murine COPD model43. Furthermore, the exaggerated CS-induced COPD-like phenotype observed in NLRX1 null mutant (–/–) mice was significantly ameliorated in NLRX1 and MAVS double-mutant (NLRX1–/–/MAVS–/–) mice, suggesting that MAVS is functioning as a critical downstream molecule during NLRX1 signaling in a murine COPD model44. Taken together, these observations allow us to speculate that NLRX1 is a critical inhibitor of CS-induced pulmonary inflammation and remodeling responses via its regulation of MAVS.
In the lung, pulmonary macrophages are major cell type that expresses NLRX1 and this is suppressed after CS exposure44. In addition, the known markers of CS-induced activation of macrophages are markedly enhanced in alveolar macrophages from CS-exposed NLRX1–/– lungs compared to those from WT controls (M.J.K, unpublished data). These results raise an exciting hypothesis that NLRX1 is an important inhibitor of CS-induced activation of macrophages. Macrophages are essential for pulmonary host defense through their capacity to survey the exposed airways and regulate innate and adaptive immunity535455. It is generally believed that alveolar macrophages are kept in a relatively quiescent state with active suppression of inflammation in response to harmless antigens to prevent collateral damage to lung tissue56. Thus, NLRX1 might keep macrophages in a quiescent homeostatic state that is disrupted by CS, which suppresses NLRX1 leading to macrophage activation (Figure 1).
We have endeavored here to illuminate connections between mitochondria and COPD that we argue could unify theories on COPD pathogenesis. Mitochondria are critical players in inflammasomes activation, ROS imbalance, apoptosis and regulation of immune responses, all of which have been implicated in COPD. The mitochondrial NRLX1 pathways we have delineated (Figure 1) might represent one type of unified hypothesis that can begin to explain the complex processes involved in COPD from a mitochondrial perspective. As such we hope it will spur further research in this rich vein to better understand and treat this currently undermet health problem.
Figures and Tables
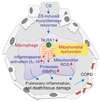
Figure 1
Mitochondrial perspective of chronic obstructive pulmonary disease (COPD) pathogenesis. Nucleotide binding domain and leucine-rich-repeat-containing protein X1 (NLRX1), which might have a crucial inhibitory role to keep alveolar macrophages (AMs) in a quiescent homeostatic status, is suppressed in patients with COPD. The suppression of NLRX1 in AMs is associated with mitochondrial dysfunction and leads to the increase of inflammasome activation, protease burden as well as production of mitochondrial reactive oxygen species (ROS), which culminate in the development of COPD. CS: cigarette smoke; IL-18: interleukin 18; MMP: matrix metalloproteinase. Please see the main text for the explanation in detail. Modified from Yoon CM, et al. J Innate Immun 2016;8:121-835, with permission of S. Karger AG, Basel.
Acknowledgements
This work was supported by Connecticut Department of Public Health Grant #2013-0196 (M.J.K.), and NIH Grant #R56HL119511 (M.J.K.).
References
1. Senior RM, Shapiro SD. Chronic obstructive pulmonary disease: epidemiology, pathophysiology, and pathogenesis. In : Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's pulmonary diseases and disorders. 3rd ed. New York: McGraw-Hill, Inc.;1998. p. 659–681.
2. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009; 4:435–459.
3. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global burden of disease and risk factors. Washington DC: The International Bank for Reconstruction and Development/The World Bank Group;2006.
4. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148:1145–1159.
5. Eriksson S. Pulmonary emphysema and alpha1-antitrypsin deficiency. Acta Med Scand. 1964; 175:197–205.
6. MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2:50–60.
7. Larsson K. Aspects on pathophysiological mechanisms in COPD. J Intern Med. 2007; 262:311–340.
8. Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011; 6:413–421.
9. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007; 176:532–555.
10. Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974; 291:755–758.
11. Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smokinginduced emphysema. Nat Med. 2007; 13:567–569.
12. Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009; 360:2445–2454.
13. Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011; 378:1015–1026.
14. Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, et al. IL-18 induces emphysema and airway and vascular remodeling via IFN-gamma, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012; 185:1205–1217.
15. Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007; 178:1948–1959.
16. Dima E, Koltsida O, Katsaounou P, Vakali S, Koutsoukou A, Koulouris NG, et al. Implication of interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD). Cytokine. 2015; 74:313–317.
17. Fischer BM, Voynow JA, Ghio AJ. COPD: balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis. 2015; 10:261–276.
18. Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art: cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006; 3:503–510.
19. Yoshida T, Tuder RM. Pathobiology of cigarette smokeinduced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87:1047–1082.
20. Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005; 25:250–258.
21. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–1217.
22. Kirkwood TB. Understanding the odd science of aging. Cell. 2005; 120:437–447.
23. Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc. 2009; 6:570–572.
24. Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013; 8:1489–1496.
25. Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015; 70:482–489.
26. Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013; 19:95–102.
27. Scheffler IE. Mitochondria. 2nd ed. Hoboken: Wiley-Liss;2007.
28. West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011; 11:389–402.
29. Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015; 42:406–417.
30. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005; 120:483–495.
31. Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest. 2016; 126:809–820.
32. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012; 481:278–286.
33. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011; 29:707–735.
34. Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140:821–832.
35. Yoon CM, Nam M, Oh YM, Dela Cruz CS, Kang MJ. Mitochondrial regulation of inflammasome activation in chronic obstructive pulmonary disease. J Innate Immun. 2016; 8:121–128.
36. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13.
37. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012; 13:780–788.
38. Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015; 163:560–569.
39. Murphy MP. Modulating mitochondrial intracellular location as a redox signal. Sci Signal. 2012; 5:pe39.
40. Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016; 61:695–704.
41. Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014; 16:728–736.
42. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005; 122:669–682.
43. Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008; 118:2771–2784.
44. Kang MJ, Yoon CM, Kim BH, Lee CM, Zhou Y, Sauler M, et al. Suppression of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest. 2015; 125:2458–2462.
45. Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O'Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013; 153:1239–1251.
46. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013; 38:225–236.
47. Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009; 122(Pt 17):3161–3168.
48. Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011; 12:901–910.
49. Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008; 451:573–577.
50. Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011; 34:854–865.
51. Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012; 36:933–946.
52. Koblansky AA, Truax AD, Liu R, Montgomery SA, Ding S, Wilson JE, et al. The innate immune receptor NLRX1 functions as a tumor suppressor by reducing colon tumorigenesis and key tumor-promoting signals. Cell Rep. 2016; 14:2562–2575.
53. Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014; 5:435.
54. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013; 14:986–995.
55. Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015; 70:1189–1196.
56. Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989; 170:499–509.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download