Abstract
A 59-year-old man presented with acute dyspnea following sudden productive cough and expectoration of a full cup of "blood-tinged" sputum. He had been diagnosed with hepatitis B virus–related hepatocellular carcinoma and had received transarterial chemoembolization 5 years ago for a 20-cm hepatic mass; he denied any history of hematemesis and the last esophagogastroduodenoscopy from a year ago showed absence of varix. Chest computed tomography (CT) with angiography showed new appearance of right basal lung consolidation but no bleeding focus. Despite the use of systemic antibiotics, the patient developed respiratory failure on day 7 of hospitalization. After intubation, a massive amount of brown sputum with anchovy-paste-like consistency was suctioned via the endotracheal tube. Bronchoscopic toileting was performed and the patient was extubated. In the ward, he continued to expectorate the brown sputum. On day 25 of hospitalization, a repeat CT scan showed simultaneous disappearance of the pneumonic consolidation and the necrotic fluid within the hepatic mass, suggesting the presence of a fistula. He has continued to receive systemic antibiotics, sorafenib, and entecavir, and follow up by respiratory and hepato-oncology specialists.
Hepatopulmonary fistula has been rarely described in the literature. Most frequently reported causes are extrahepatic biliary duct obstruction due to benign strictures or stones, causing bronchobiliary fistula due to increased pressure or procedure-related complications123. However, spontaneous fistula formation between hepatocellular carcinoma (HCC) and the lung parenchyma is very rare. HCC is a common malignancy in Asia and is the third leading cause of cancer mortality worldwide. Spontaneous HCC ruptures are often fatal since most patients present with hemoperitoneum or hemorrhagic shock456; non-bleeding HCC rupture has been reported in the literature only once and occurred within the peritoneal cavity7. Here, we report a case of necrotizing pneumonia due to spontaneous rupture and drainage of a huge, necrotic HCC into the lung parenchyma and bronchus.
A 59-year-old man presented to the Emergency Department (ED) with acute progressive dyspnea preceded by expectoration of a cup of "blood-tinged" sputum. The "hemoptysis" had started 3 hours prior after sudden coughing and was described by his wife as very dark in color. Our electronic medical record indicated that he had attended our outpatient hepatology clinic regularly for hepatitis B virus–related HCC with a huge 20-cm mass in the right hemiliver, which was diagnosed 5 years ago; he received transarterial chemoembolization (TACE) twice for the mass at the time of diagnosis and no further invasive procedure was performed for the past years; follow-up abdominal imaging regularly at 6-month interval showed no evidence of tumor rupture and no change of necrotic mass until recently (Figure 1). Current medications included sorafenib and entecavir, which he had taken for the past 5 years. He denied any history of aspiration, vomiting, hematemesis, or melena; the last esophagogastroduodenoscopy did not show presence of varix a year ago. Recently, he had intermittent right flank discomfort and a low-grade fever without chills for a year. He was a never-smoker and denied history of asthma.
On arrival, he was in acute respiratory distress with a respiratory rate of 28 breaths per minute and was receiving oxygen at the rate of 10 L/min via a facial mask in the ambulance. Other vital signs were as follows: blood pressure 138/91 mm Hg, pulse rate 111 beats per minute, temperature 36.4℃, and oxygen saturation 90% using the full facial oxygen mask. Bilateral whole-lung wheezing was dominant and right-sided crackles were heard upon auscultation. There was no visible blood in the oral cavity. The results of the initial arterial blood gas analyses during full facial oxygen mask administration were pH 7.4, pCO2 30 mm Hg, pO2 79 mm Hg, and HCO3 20.4 mmol/L. After administering an inhaled bronchodilator with a systemic steroid, intravenous methylprednisolone 40 mg, his oxygen demand gradually decreased to 3 L/min administered via a nasal cannula.
In order to find the possible source of the "hemoptysis," computed tomography (CT) of the chest with angiography was performed, which showed a new area of consolidation in the right basal lung suggesting necrotizing pneumonia (Figure 1). Otherwise, no definite hypertrophied bronchial artery was seen and there was no possible bleeding focus.
Blood tests showed marked leukocytosis (28,690/mL, 78% segmented neutrophils) with significantly elevated C-reactive protein (CRP, 28.11 mg/dL) and lactic acid (4.3 mmol/L).
There was no change in the hemoglobin level from his baseline (12.8 g/dL). His hematocrit was 38.3% and the platelet count was normal (252×103/µL). The blood urea nitrogen was not elevated (15 mg/dL); serum sodium was 134 mmol/L and the serum glucose level was 158 mg/dL. Liver function tests were also within the usual range (aspartate aminotransferase, 35 IU/L; alanine aminotransferase, 40 IU/L; total bilirubin, 1.1 mg/dL; albumin, 3.3 g/dL; and prothrombin time, 64% [patient's baseline, 69%]).
Treatment with intravenous piperacillin/tazobactam 4.5 g every 6 hours and levofloxacin 750 mg daily was initiated immediately and the patient was admitted to the general ward. Given the relatively mild pneumonia severity index (risk class III, 89 points) and radiologic extent of pneumonia, inflammatory markers including leukocyte count and CRP were persistently highly elevated. There were no improvements in his clinical signs (respiratory symptoms, cough, dark-colored sputum, and wheezing) or X-ray while continuing antibiotics. There was no bacterial growth on the initial blood and sputum cultures. A sputum acid fast stain for tuberculosis was negative. The urinary pneumococcal antigen test was also negative. On hospitalization day 5, his dyspnea worsened and the oxygen demand increased up to 6 L/min via nasal cannula.
On hospital day 7, the patient developed type 2 respiratory failure (pH, 7.13; pCO2, 105 mm Hg; pO2, 123 mm Hg; HCO3, 25.2 mmol/L) despite the use of noninvasive ventilator support overnight. Intubation was performed, and immediately afterwards, a massive volume of dark-brown secretions with a consistency like anchovy-paste was expectorated via the endotracheal tube. We thought that it was very strange because his mental status had been alert and there was no preceding aspiration event. Since the huge hepatic mass had been compressing the right lung, we considered the possibility of a connection and reviewed the initial CT scan with the Division of Thoracic Imaging, Department of Radiology. Indeed, a possible communication tract between the hepatic mass and the right middle bronchus was discovered (Figure 1). A sputum smear was performed which showed numerous neutrophils and dyskeratotic cells.
The patient was admitted to the intensive care unit to receive mechanical ventilation and the antibiotic therapy was changed to intravenous meropenem 1 g every 8 hours. Bronchoscopy performed the next day showed obstruction of the right middle bronchus with a large amount of thick brown secretions. No overt defect was seen. After toileting, the patient was successfully extubated.
In the ward, he continued to expectorate dark brown sputum despite improvement in the pneumonic consolidation, laboratory findings, and oxygen demand. After 12 days of intravenous antibiotics to cover necrotizing pneumonia and continued administration of sorafenib and entecavir, the chest X-ray showed slightly improved consolidation and a sign of an air-fluid level under the right diaphragm.
On the 25th hospital day, a repeat CT scan was taken, which showed simultaneous disappearance of the pneumonic consolidation and the necrotic fluid within the hepatic mass (Figure 2). A fistula connecting the two sides was seen (Figure 3). The patient's history was carefully recorded again and the patient and his wife confirmed that there had not been any blood in the sputum at any time; it had always been brown and like the anchovy-paste (Figure 4). It turned out that an intern in the ED had initially recorded it as "hemoptysis." A sputum smear was performed again and showed numerous bilirubin castlike objects (Figure 4).
Considering the short life expectancy due to HCC, he and his family did not want to receive further invasive procedures or surgery. While continuing antibiotics and tapering the steroid, the patient received inpatient rehabilitation and was successful discharged. We decided to continue antibiotics, sorafenib, and entecavir in the outpatient clinic. He is now followed-up by both respiratory physicians and hepatologists for 1 month without recurrence of pneumonia.
Based on our knowledge, this is the first case where necrotic fluid within a giant HCC spontaneously spilled into the lung parenchyma resulting in necrotizing pneumonia. Baudet et al.1 described a similar, but different case of a bronchobiliary fistula secondary to the invasion of bile ducts by a HCC. In that case, the initial presentation was obstructive jaundice and bile was present in the sputum, which was suggested to be pathognomonic.
It was unique that our patient was not icteric and the serum bilirubin level was normal; there was no sign of prior bile flow obstruction. Rather, it was the necrotic fluid within the HCC, not bile, which ruptured into the pleura and the lung parenchyma, forming a possible hepatopulmonary fistula in this case. The patient's brown sputum, which resembled anchovypaste, was similar to those found in hepatopulmonary fistulas caused by amoebic abscesses as reported in previous studies. The reason for this similarity can be attributed to possible mechanism where prior TACE, which was done 5 years ago, may have resulted in necrosis of the huge HCC; current use of sorafenib may helped in generating a pus-filled cavity, resembling hepatic abscess, and as the extensive suppurative process may have led to erosion of diaphragm and rupture into the pleural space and pulmonary parenchyma2.
In this case, percutaneous catheter drainage of necrotic fluid along with the administration of systemic antibiotics with gram-negative and anaerobe coverage could also be considered as treatment options. However, since the patient's condition markedly improved with the current medical treatment and expectoration, we chose not to insert the catheter due to quality of life concerns and the patient's wish.
Figures and Tables
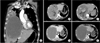 | Figure 1(A) Initial computed tomography (CT) of chest taken at the Emergency Department in June 2015 shows new appearance of right basal lung consolidation but no focus of hemoptysis. (B-E) Review of previous abdominal imaging shows necrotic change of the very large hepatocellular carcinoma (HCC). (B) The patient was diagnosed with HCC 5 years ago in October 2010; note a 20-cm sized mass on the right hemiliver. (C) In November 2010, he had undergone transarterial chemoembolization (TACE) via S1, S7, right and left hepatic arteries, and both inferior phrenic arteries. Lipiodol CT scan after TACE shows extensive necrosis of the HCC without evidence of rupture. (D, E) The patient received second TACE in July 2011 and additional lipiodol uptakes along margin of necrotic mass in right lobe were noted; however, there was no evidence of tumor rupture. Regular follow-up abdominal CT scans at 6 month intervals showed no further change of the very large necrotic lesion (the images shown are of January 2013 and April 2015, respectively). Meanwhile, the patient received stereotactic ablative radiotherapy to S1 bone metastasis in May 2015, and no further invasive procedure was performed. He has continued to take sorafenib and entecavir since December 2010. |
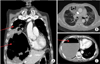 | Figure 2(A–C) Repeat computed tomography scan of the chest and abdomen on day 25 of hospitalization. Note slight improvement of pneumonic consolidation and spontaneous disappearance of some necrotic fluid within the giant hepatocellular carcinoma (HCC) (upper and lower arrow in A, respectively). (C) New appearance of air-fluid level within the necrotic HCC (arrow). |
References
1. Baudet JS, Medina A, Moreno A, Navazo L, Aviles J, Soriano A. Bronchobiliary fistula secondary to ruptured hepatocellular carcinoma into the bile duct. J Hepatol. 2004; 41:1066–1067.
2. Strange C, Allen ML, Freedland PN, Cunningham J, Sahn SA. Biliopleural fistula as a complication of percutaneous biliary drainage: experimental evidence for pleural inflammation. Am Rev Respir Dis. 1988; 137:959–961.
3. Yoon DH, Shim JH, Lee WJ, Kim PN, Shin JH, Kim KM. Percutaneous management of a bronchobiliary fistula after radiofrequency ablation in a patient with hepatocellular carcinoma. Korean J Radiol. 2009; 10:411–415.
4. Ono F, Hiraga M, Omura N, Sato M, Yamamura A, Obara M, et al. Hemothorax caused by spontaneous rupture of hepatocellular carcinoma: a case report and review of the literature. World J Surg Oncol. 2012; 10:215.
5. Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006; 141:191–198.
6. Kim JS, Yoon SK, Kim JA, Kim CW, Bae SH, Choi JY, et al. Long-term survival in a patient with ruptured hepatocellular carcinoma. Korean J Intern Med. 2009; 24:63–67.
7. Islam M, Deka P, Kapur R, Ansari MA. Non-bleeding spontaneous rupture of hepatocellular carcinoma. Niger J Surg. 2013; 19:82–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download