Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive and lethal lung disease characterized by the accumulation of excessive fibroblasts and myofibroblasts in the extracellular matrix. The transforming growth factor β1 (TGF-β1)–induced epithelial-to-mesenchymal transition (EMT) is thought to be a possible source of fibroblasts/myofibroblasts in IPF lungs. We have previously reported that apolipoprotein A1 (ApoA1) has anti-fibrotic activity in experimental lung fibrosis. In this study, we determine whether ApoA1 modulates TGF-β1–induced EMT in experimental lung fibrosis and clarify its mechanism of action.
Methods
The A549 alveolar epithelial cell line was treated with TGF-β1 with or without ApoA1. Morphological changes and expression of EMT-related markers, including E-cadherin, N-cadherin, and α-smooth muscle actin were evaluated. Expressions of Smad and non-Smad mediators and TGF-β1 receptor type 1 (TβRI) and type 2 (TβRII) were measured. The silica-induced lung fibrosis model was established using ApoA1 overexpressing transgenic mice.
Results
TGF-β1–treated A549 cells were changed to the mesenchymal morphology with less E-cadherin and more N-cadherin expression. The addition of ApoA1 inhibited the TGF-β1–induced change of the EMT phenotype. ApoA1 inhibited the TGF-β1–induced increase in the phosphorylation of Smad2 and 3 as well as that of ERK and p38 mitogen-activated protein kinase mediators. In addition, ApoA1 reduced the TGF-β1–induced increase in TβRI and TβRII expression. In a mouse model of silica-induced lung fibrosis, ApoA1 overexpression reduced the silica-mediated effects, which were increased N-cadherin and decreased E-cadherin expression in the alveolar epithelium.
Idiopathic pulmonary fibrosis (IPF) is a progressive, lethal lung disease characterized by alveolar epithelial cell injury, proliferation of activated fibroblasts and myofibroblasts and extracellular matrix accumulation/remodeling that leads to irreversible distortion of the lung parenchyma12.
It has been suggested that the origin of the activated fibroblasts and myofibroblasts are from several, but not mutually exclusive, pathways. Traditionally, migration/proliferation of the resident fibroblasts is thought to be a major source of activated fibroblasts and myofibroblasts3. Recently, it has been shown that activated fibroblasts and myofibroblasts could arise from other cells like bone marrow-derived circulating fibrocytes4, microvascular pericytes5, endothelial cells6, and alveolar epithelial cells (AECs)78.
Epithelial-to-mesenchymal transition (EMT) is highlighted as an important, possible mechanism of fibrosis within various organs7910. There is evidence in which cuboidal epithelial cells, originally possessing the epithelial cell marker, are transformed to fibroblast-like spindle cell morphology with new expression of the mesenchymal cell marker after transforming growth factor β1 (TGF-β1) treatment, and cells co-expressing both the epithelial and mesenchymal markers are seen in the human IPF lung as well as TGF-β1–treated AECs911. The histological evidence implies that EMT is a functional transition of polarized epithelial cells into migratory mesenchymal cells, suggesting that the origin of the activated fibroblasts and myofibroblasts are the epithelial cells that are injured and follow an aberrant healing process, losing their normal epithelial regenerative capacity9.
Injured epithelial cells prompt the fibrogenic process by releasing TGF-β1, which is a prototypical profibrotic growth factor and well known for having a pivotal role in inducing EMT, commonly throughout all organs10. TGF-β1 expression is up-regulated in the IPF patient's lungs, especially in bronchiolar and AECs, and the extent of adjacent subepithelial TGF-β1 deposition is associated with fibroblast proliferation/accumulation 12.
Currently, there are no proven drugs that cure IPF. Antiinflammatory drugs such as azathioprine and prednisone have no effect on slowing the progression of lung fibrosis and show no survival benefit1314. Meanwhile, anti-fibrotic agents such as nintedanib and pirfenidone have been proven to slow progression of IPF14.
Recently, we reported that apolipoprotein A1 (ApoA1) has an anti-inflammatory and anti-fibrotic effect on the bleomycin and silica-induced lung fibrosis model1516. Lung ApoA1 expression is decreased in IPF as well as in the bleomycininduced fibrotic mouse, and intranasal ApoA1 treatment has been shown to attenuate lung inflammation and fibrosis in the mouse model15. In sequence, we also demonstrated that ApoA1 overexpression attenuated silica-induced established lung fibrosis by using an ApoA1 transgenic mouse model16. Although, our previous data represented anti-inflammatory and anti-fibrotic activities of ApoA1, we could not evaluate the precise mechanism of its anti-fibrotic activities, especially in EMT.
In this study, we have investigated whether ApoA1 modulates TGF-β1–induced EMT and have explored possible mechanisms using the AEC line and the silica-induced lung fibrosis mouse model.
A549 cells (ATCC CCL185; American Type Culture Collection, Manassas, VA, USA) were obtained from the ATCC and maintained in Ham's F12K medium with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. To induce EMT, cells were stimulated for 48 hours in serum free medium (SFM) with 0.1% bovine serum albumin (BSA) and TGF-β1 (5 ng/mL, R&D Systems, Minneapolis, MN, USA). To evaluate the effect of ApoA1, the culture medium was replaced with SFM supplemented with 0.1% BSA, TGF-β1 (5 ng/mL) and recombinant human ApoA1 (100 ng/mL, Calbiochem, San Diego, CA, USA) and the cells were incubated for 48 hours. Control cells were maintained in SFM with 0.1% BSA for 48 hours. The cellular morphological change was evaluated using phase control microscopy (Carl Zeiss Microsystems, Thornwood, NY, USA). Western blot and immunofluorescence were used to measure the expression of N-cadherin, E-cadherin, and α-smooth muscle actin (α-SMA). Smad and non-Smad pathway related molecules and TGF-β1 receptor protein (type I [TβRI] and II [TβRII]) expression were measured by western blot in the cell.
Male ApoA1 overexpressing transgenic mice (6–8 weeks old) were treated with silica intratracheally following our previously described method16. On day 0, the transgenic mice received 20 mg of sterile silica crystals (median diameter, 1–5 µm; Sigma-Aldrich, St. Louis, MO, USA) in endotoxin-free water in a total volume of 100 µL by intratracheal delivery. ApoA1 overexpressing transgenic and ApoA1 non-overexpressing wild type mice were housed and sacrificed on day 30. Lung sections were subjected to immunofluorescence staining with confocal microscopy (LSM 510 META; Carl Zeiss Microsystems).
For western blot analysis, A549 cells were prepared by extracting proteins with RIPA buffer (50 mM Tris-HCl [pH 7.4], 1% [V/V] NP-40, 150 mM NaCl, and 1 mM ethylenediaminetetraacetic acid) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 µM aprotinin, 1 µg/mL leupeptin, and 1 mM Na3VO4). Lung tissue was homogenized in RIPA buffer containing protease inhibitors. Equal amounts of proteins were resolved using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). After blocking with non-fat dried milk, the membranes were incubated with primary antibodies for 1 hour at room temperature followed by detection using horseradish peroxidase conjugated secondary antibodies. Enhanced chemiluminescence detection was performed according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany). The relative abundance of protein was determined by quantitative densitometry using Image J software (NIH, Bethesda, MD, USA). All western blot densitometry data were normalized to β-actin.
For immunofluorescence analysis, mouse lung tissue was incubated at 4℃ overnight with anti E-cadherin (1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and N-cadherin antibodies (1:1,000, BD Bioscience, Bedford, MA, USA). Fluorescein isothiocyanate-conjugated donkey anti-goat antibodies (1:1,000, Santa Cruz Biotechnology Inc.) and goat anti-rabbit IgG-PE (1:1,000, Santa Cruz Biotechnology Inc.) were used as secondary antibodies for the localization of E-cadherin and N-cadherin in the mouse lung.
The antibodies used in the present study included anti-human E-cadherin (1:500, Santa Cruz Biotechnology Inc.), anti-human N-cadherin (1:1,000, BD Bioscience), anti-α-SMA (1:300, Abcam, Cambridge, UK), anti–β-actin (1:5,000, Sigma-Aldrich), anti-Smad3, anti-pSmad3 (1:1,000, Cell Signaling Technology, Danvers, MA, USA), anti-ERK1/2, anti-pERK1/2 (1:1,000, Cell Signaling Technology), anti-p38 mitogen-activated protein kinase (MAPK), anti-pp38 MAPK (1:1,000, Cell Signaling Technology), anti-TβRI (1:1,000, Santa Cruz Biotechnology Inc.), and anti-TβRII (1:500, Abcam).
As previously reported, in response to TGF-β1, A549 cells were converted from an epithelial phenotype to a spindle-like mesenchymal phenotype, and treatment with ApoA1 restored the cells to the traditional polygonal epithelial shape (Figure 1). As expected, exposing the cells to TGF-β1 for 48 hours showed a reduced expression of the epithelial marker (E-cadherin) with increased expression of the mesenchymal marker (N-cadherin and α-SMA). These phenotypic changes were inhibited by treatment of ApoA1 (Figures 2, 3). Silica-treated mouse lung showed decreased E-cadherin and increased N-cadherin expression in the alveolar epithelium, shown as the pro-surfactant protein C, and overexpression of ApoA1 inhibited the changes in the epithelial and mesenchymal markers in the alveolar epithelium (Figure 4). These findings demonstrate that ApoA1 effectively inhibits both TGF-β1–induced EMT in the AEC and silica-induced EMT in vivo.
We determined whether ApoA1 interferes with the TGF-β1 signaling pathways. We examined whether the inhibitory effects of ApoA1 against TGF-β1–induced EMT in A549 cells are mediated by changing the phosphorylated Smad2 or Smad3 expression, which have been demonstrated to be the major signal pathways in TGF-β1–induced EMT17.
Phosphorylation of Smad2 and Smad3 in A549 cells was markedly increased in a time-dependent manner by TGF-β1 treatment. When the cells were treated with ApoA1, phosphorylation of Smad2 and Smad3 were significantly reduced (Figure 5). This finding suggests that ApoA1 inhibits the Smad-dependent TGF-β1 signaling pathway. TGF-β1 also induced non-Smad responses, including ERK and p38 MAPK mediated signaling18. ApoA1 also reduced the phosphorylation of ERK and p38 MAPK induced by TGF-β1 (Figure 6). It appears that ApoA1 down-regulates both Smad-dependent and non-Smad signaling pathways induced by TGF-β1.
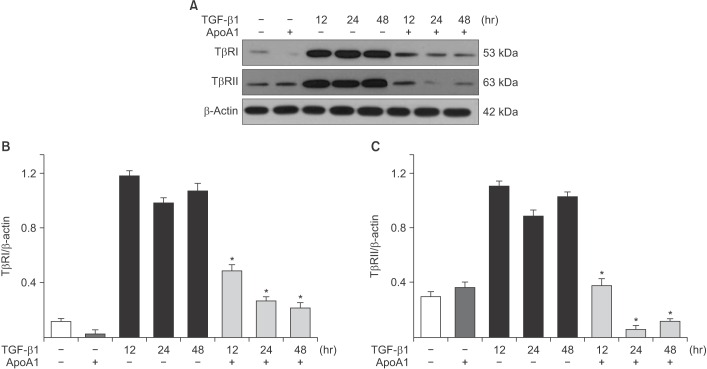
TGF-β1 signaling is initiated by the binding of TGF-β1 to TβRI and TβRII on the epithelial cell membrane19. Increases in the TβRI and TβRII protein expression levels were observed in A549 cells upon TGF-β1 treatment, whereas the addition of ApoA1 significantly reduced the expression of these proteins (Figure 7).
In this study, we have shown that ApoA1 effectively inhibits TGF-β1–induced EMT, both in vitro and in vivo. ApoA1 remarkably reduced TGF-β1–induced expression of mesenchymal markers such as α-SMA and N-cadherin and recovered the expression of the epithelial marker E-cadherin in AECs (Figures 2, 3). We also observed that ApoA1 overexpression inhibits silica-induced EMT in the mouse model (Figure 4).
In the currently accepted paradigm, the main pathogenesis of IPF is thought to be recurrent AEC injury that leads to aberrant activation of AEC, creating the accumulation of collagen producing fibroblasts and myofibroblasts2021. Although the significance and relative contribution of EMT to the source of activated fibroblasts and myofibroblasts has not been definitely established, EMT has been increasingly proposed as one of the main mechanisms of excessive fibroblast proliferation in lung fibrosis2223. For the induction of EMT in vitro and in vivo, TGF-β1 is regarded as the prototypical cytokine24. TGF-β1–induced EMT starts with the activation of latent TGF-β1, which originally existed in the extracellular mileu, kept inactive by the latency-associated protein, and bound by the latent TGF binding protein. Then, TGF-β1 binds with the TβRII and TβRI, activating the receptor heterodimer and initiating the intracellular Smad and non-Smad signal pathways24.
TGF-β1 signaling occurs via both Smad dependent and non-dependent pathways25. The Smad dependent signal transduction system is the main process in the TGF-β1 signaling. When the extracellular TGF-β ligand binds to the cell membrane TGF-β receptor, the receptor-activated Smads (R-SMADS), Smad2 and 3, are phospholylated directly by the intracellular TβRI kinase domain, and then bind to the common mediator Smad (Co-SMAD), Smad4. These Smad proteins translocate into the nucleus, bind with Smad-binding elements as a transcription factor complex, and activate TGF-β1 target genes (e.g., α-SMA and collagen), as well as inhibiting epithelial genes (e.g., E-cadherin) through the co-association of various transcription factors1724. For the full TGF-β1 response, phosphoinositide 3-kinase/Akt or MAPK-targeted transcription factors bind to the critical response element of the TGF-β target genes in the Smad independent pathway. The ERK/MAPK cascade additionally phosphorylates R-SMADS, modifying Smad activity and enhances TGF-β1–mediated collagen I synthesis26. Besides such interaction with the Smad pathway in modulation and activation of transcription factors, non-Smad proteins are also involved in a variety of cellular responses, including cellular tight/adheren junction disassembly, and cytoskeletal rearrangement2425.
Each step in the TGF-β1–induced EMT signal pathway has been considered as a possible, effective therapeutic target for lung fibrosis27. Several studies to identify the proper therapeutic target in the TGF-β1–induced EMT signal pathway have been completed or are in progress2228293031. However, currently, there is no therapeutic agent that directly inhibits TGF-β1, due to its complex function and the expected side effects of direct TGF-β1 inhibition28.
In this study, treatment of ApoA1 attenuated TGF-β1–induced Smad2 and Smad3 phosphorylation in A549 cell (Figure 5). Moreover, ApoA1 also inhibited non-Smad signaling, particularly the ERK and MAPK pathways (Figure 6). Since both Smad-dependent and non-Smad pathways were inhibited by ApoA1 in our study, we evaluated further whether ApoA1 modulates TGF-β receptor expression. Rojas et al.32 reported that the TGF-β receptor levels regulate the specificity of the signaling pathway activation and the biologic effects of TGF-β1 and, in particular, that the TβRII expression levels are correlated with Smad signaling and MAPK-ERK signaling activation. We have shown that ApoA1 inhibits both TβRI and TβRII expression induced by TGF-β1 (Figure 7). The exact molecular mechanism by which ApoA1 modulates the expression of TGF-β receptors remains to be determined. We speculate that ApoA1 interferes with the binding or interaction between the TGF-β1 ligand and the TβRI-TβRII complex, resulting in down-regulation of downstream signaling molecules.
We examined the anti-EMT activity of ApoA1 through the murine model of silica-induced lung fibrosis using a previously reported method16. ApoA1 transgenic mice and a silica-induced lung fibrosis model were used because even distribution of ApoA1 in the alveolar epithelium and permanent chronic lung fibrosis are essential for EMT experiments in vivo. In our previous data, active TGF-β1 levels in the lung were significantly increased in the silica-treated mice16. In the present study, intratracheal treatment of silica decreased E-cadherin and increased N-cadherin expression in the alveolar epithelium. Overexpression of ApoA1 inhibits these EMT-related phenotypic marker changes. Taken together, we have demonstrated that ApoA1 blocks TGF-β1–induced EMT both in vitro and in vivo.
ApoA1 was originally known as the major apolipoprotein composed of high density lipoprotein cholesterol. In addition to cholesterol modulation, ApoA1 has been shown to possess anti-inflammatory activity3334. Recently, we reported that ApoA1 has a therapeutic potential on a bleomycin and silicainduced murine model of fibrosis, with possible mechanisms associated with anti-apoptosis, anti-oxidative activity and the ability to promote the generation of pro-resolutional mediators such as lipoxin A4151635. In addition to these results, these data support the idea that the inhibition of EMT by ApoA1 may at least partly contribute to its anti-fibrotic activity in the experimental lung fibrosis model. Further study is needed to investigate how ApoA1 modulates TGF-β receptor expression.
In conclusion, this study demonstrates that ApoA1 inhibits TGF-β1–induced EMT in AECs and a silica-induced lung fibrosis animal model. ApoA1 down-regulates Smad-dependent and non-Smad TGF-β1 signaling pathways and reduces TβRI and TβRII expression. These findings could be helpful in understanding the therapeutic effect of ApoA1 on lung fibrosis.
Acknowledgements
This research was supported by the 2012 grant from The Korean Academy of Tuberculosis and Respiratory Diseases. And by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (Grant 2014R1A2A2A01007383).
References
1. Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/ fibroblastic cross-talk disorder. Respir Res. 2002; 3:3. PMID: 11806838.

3. Ross R, Everett NB, Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol. 1970; 44:645–654. PMID: 5415241.
4. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994; 1:71–81. PMID: 8790603.

5. Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagenproducing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008; 173:1617–1627. PMID: 19008372.

6. Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010; 43:161–172. PMID: 19767450.

7. Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004; 15:1–12. PMID: 14694152.

8. Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005; 6:56. PMID: 15946381.

9. Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006; 3:377–382. PMID: 16738204.

10. Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007; 282:23337–23347. PMID: 17562716.

11. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005; 166:1321–1332. PMID: 15855634.
12. Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991; 5:155–162. PMID: 1892646.
13. Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012; 366:1968–1977. PMID: 22607134.
14. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015; 192:e3–e19. PMID: 26177183.

15. Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010; 182:633–642. PMID: 20463180.
16. Lee Eh, Lee EJ, Kim H, Jang A, Koh E, Uh ST, et al. Overexpression of apolipoprotein A1 in the lung abrogates fibrosis in experimental silicosis. PLoS One. 2013; 8:e55827. PMID: 23409054.

17. Heldin CH, Moustakas A. Role of Smads in TGFbeta signaling. Cell Tissue Res. 2012; 347:21–36. PMID: 21643690.
18. Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012; 347:11–20. PMID: 21701805.

19. Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009; 21:166–176. PMID: 19237272.
20. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011; 378:1949–1961. PMID: 21719092.

21. Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, et al. Idiopathic pulmonary fibrosis: an update. Ann Med. 2015; 47:15–27. PMID: 25613170.

22. Noguchi S, Yamauchi Y, Takizawa H. Novel therapeutic strategies for fibrotic lung disease: a review with a focus on epithelial-mesenchymal transition. Recent Pat Inflamm Allergy Drug Discov. 2014; 8:9–18. PMID: 24383438.

23. Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014; 69:760–765. PMID: 24334519.

24. Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L525–L534. PMID: 17631612.
25. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–584. PMID: 14534577.
26. Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003; 17:1576–1578. PMID: 12824291.
27. Lee CM, Park JW, Cho WK, Zhou Y, Han B, Yoon PO, et al. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med. 2014; 29:281–290. PMID: 24851060.
28. Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007; 132:1311–1321. PMID: 17934117.
29. Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009; 16:1400–1417. PMID: 19355895.
30. Gomer RH, Lupher ML Jr. Investigational approaches to therapies for idiopathic pulmonary fibrosis. Expert Opin Investig Drugs. 2010; 19:737–745.

31. Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011; 163:141–172. PMID: 21265830.

32. Rojas A, Padidam M, Cress D, Grady WM. TGF-beta receptor levels regulate the specificity of signaling pathway activation and biological effects of TGF-beta. Biochim Biophys Acta. 2009; 1793:1165–1173. PMID: 19339207.
33. Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010; 18:141–147. PMID: 20395699.
34. Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT. Apolipoprotein mimetic peptides: mechanisms of action as antiatherogenic agents. Pharmacol Ther. 2011; 130:83–91. PMID: 21172387.

35. Park SW, Lee EH, Lee EJ, Kim HJ, Bae DJ, Han S, et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin Exp Allergy. 2013; 43:914–927. PMID: 23889245.

Figure 1
ApoA1 inhibits TGF-β1–induced morphological change in A549 cells. A549 cells were incubated with TGF-β1 (5 ng/mL) with or without ApoA1 (100 ng/mL) for 48 hours. (A) Untreated A549 cells showed a polygonal shape and intact cell-cell adhesion. (B) TGF-β1–treated cells showed a decrease in cell-cell adhesion and a greater spindle shape. (C) Co-treatment of ApoA1-treated cells maintained a polygonal shape and showed an intact cell-cell adhesion similar to the untreated cells (A–C, ×400). ApoA1: apolipoprotein A1; TGF-β1: transforming growth factor β1.
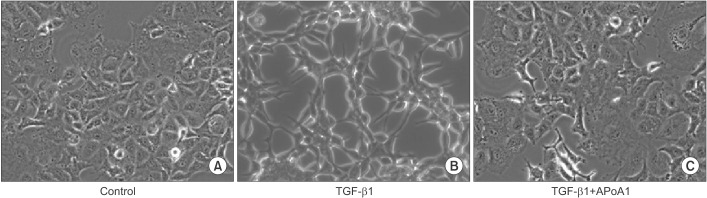
Figure 2
ApoA1 inhibits TGF-β1–induced EMT in A549 cells. A549 cells were treated with TGF-β1 (5 ng/mL) with or without ApoA1 (100 ng/mL) for 48 hours. Expressions of E-cadherin (green) (A), N-cadherin (green) (B), and α-SMA (red) were analyzed by immunofluorescence staining. Treatment with TGF-β1 decreased the expression of the epithelial marker, E-cadherin, and increased the expression of mesenchymal markers such as N-cadherin and α-SMA. ApoA1 inhibited TGF-β1–induced changes in EMT markers similar to control levels (A and B, ×400). ApoA1: apolipoprotein A1; TGF-β1: transforming growth factor β1; EMT: epithelial-to-mesenchymal transition; α-SMA: α-smooth muscle actin; DIC: differential interference contrast.
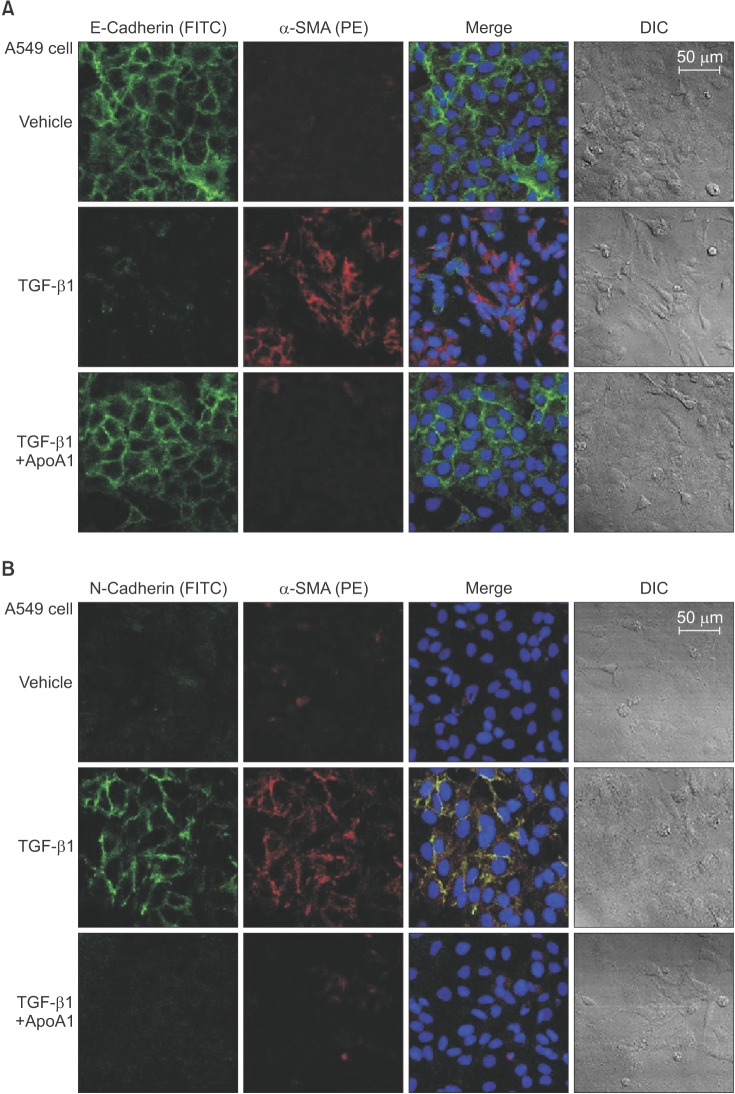
Figure 3
Effects of ApoA1 on EMT-related markers in A549 cells. A549 cells were incubated with TGF-β1 (5 ng/mL) in the absence of serum with or without ApoA1 (100 ng/mL) for up to 48 hours. Stimulation of cells by TGF-β1 down-regulated the epithelial marker, E-cadherin, and up-regulated the mesenchymal markers such as N-cadherin and α-SMA in a time dependent manner. β-Actin was used as a loading control (A). Densitometric analysis of band intensities for E-cadherin (B), N-cadherin (C), and α-SMA band (D). Each bar represents mean±standard error of at least three independent experiments. *p<0.05 versus same time of the TGF-β1–treated group. ApoA1: apolipoprotein A1; EMT: epithelial-to-mesenchymal transition; TGF-β1: transforming growth factor β1; α-SMA: α-smooth muscle actin.
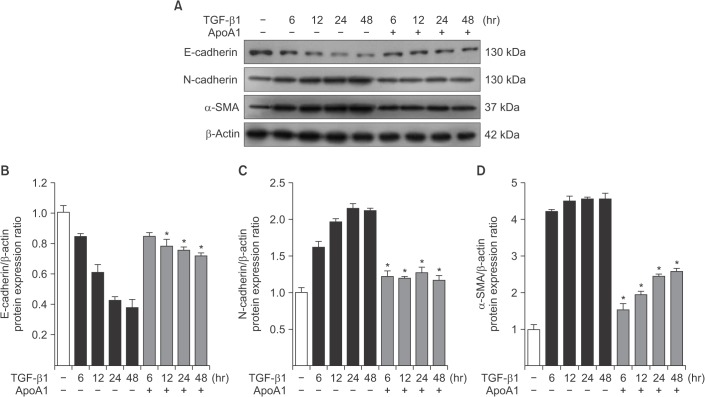
Figure 4
Overexpression of ApoA1 attenuates silica-induced EMT in the mouse lung. Immunofluorescence staining for E-cadherin (green) (A), N-cadherin (green) (B), and pro-SPC (red) in the mouse lung. Treatment with silica decreased the expression of the epithelial marker, E-cadherin, and increased the expression of the mesenchymal markers such as N-cadherin and α-SMA in the WT mouse lung. Overexpression of ApoA1 inhibited TGF-β1–induced changes in EMT markers similar to silica non-treated WT levels (first-fourth columns, ×1,000; fifth columns, ×400). ApoA1: apolipoprotein A1; EMT: epithelial-to-mesenchymal transition; SPC: surfactant protein C; α-SMA: α-smooth muscle actin; WT: wild type; TGF-β1: transforming growth factor β1; DIC: differential interference contrast; TG: transgenic.
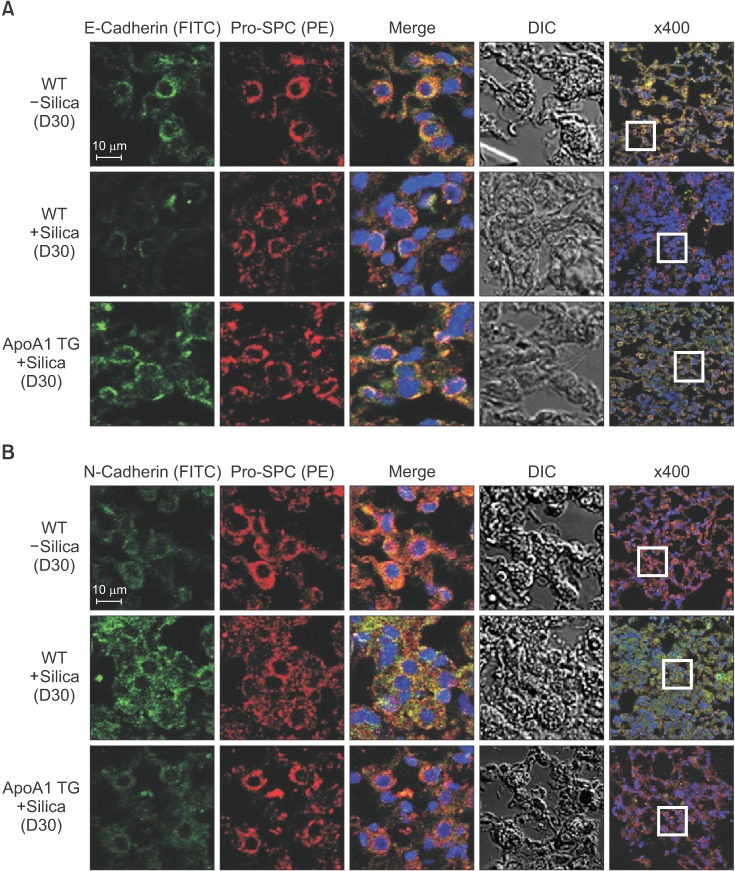
Figure 5
ApoA1 down-regulates the TGF-β1–induced Smad dependent signaling pathway. A549 cells were incubated with TGF-β1 (5 ng/mL) with or without ApoA1 (100 ng/mL) for 48 hours. (A) Phosphorylation of Smad2 and Smad3 occurred after TGF-β1 stimulation and co-treatment with ApoA1 down-regulated the TGF-β1–induced Smad signaling pathway. β-Actin was used as a loading control. Densitometric analysis of band intensities for phosphorylated Smad2 (B) and Smad3 (C). Each bar represents mean±standard error of at least three independent experiments. *p<0.05 versus same time of the TGF-β1–treated group. ApoA1: apolipoprotein A1; TGF-β1: transforming growth factor β1.
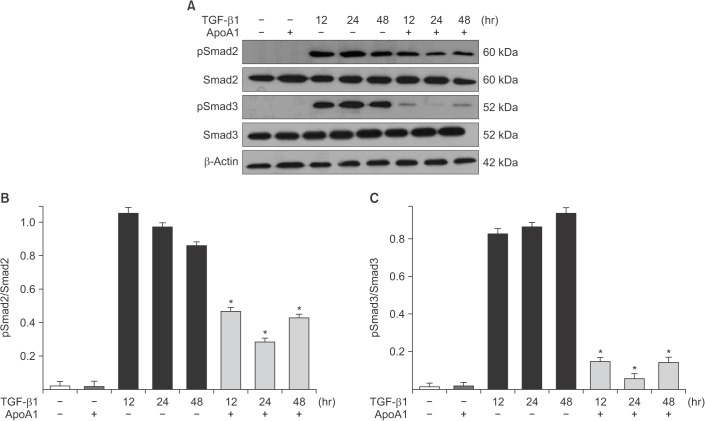
Figure 6
ApoA1 down-regulates the TGF-β1–induced non-Smad signaling pathways. A549 cells were incubated with TGF-β1 (5 ng/mL) with or without ApoA1 (100 ng/mL) for 48 hours. (A) Phosphorylation of ERK1/2 and p38 MAPK occurred after TGF-β1 stimulation and co-treatment with ApoA1 down–regulated the TGF-β1–induced non-Smad signaling pathways. β-Actin was used as a loading control. Densitometric analysis of band intensities for phosphorylated ERK1/2 (B) and p38 MAPK (C). Each bar represents mean±standard error of at least three independent experiments. *p<0.05 versus same time of the TGF-β1–treated group. ApoA1: apolipoprotein A1; TGF-β1: transforming growth factor β1; MAPK: mitogen-activated protein kinase.
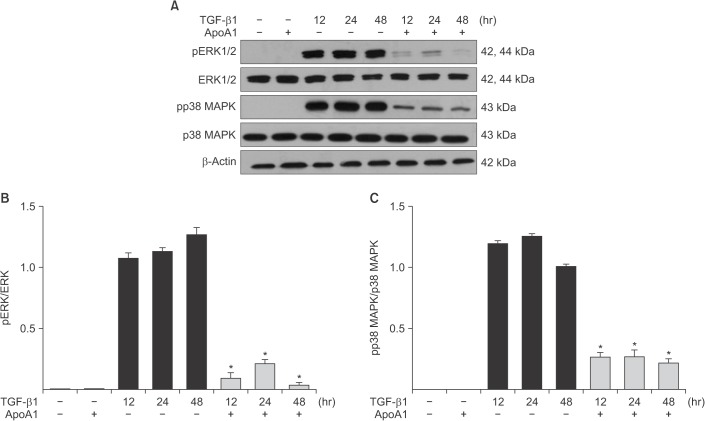
Figure 7
ApoA1 decreases the expression of TβRI and TβRII in A549 cells. A549 cells were incubated with TGF-β1 (5 ng/mL) with or without ApoA1 (100 ng/mL) for 48 hours. (A) TGF-β1 increased both TβRI and TβRII expression and co-treatment with ApoA1 down-regulated TGF-β receptors. β-Actin was used as a loading control. Densitometric analysis of band intensities for TβRI (B) and TβRII (C). Each bar represents mean±SE of at least three independent experiments. *p<0.05 versus same time of the TGF-β1–treated group. ApoA1: apolipoprotein A1; TGF-β1: transforming growth factor β1; TβRI: TGF-β1 receptor type 1; TβRII: TGF-β1 receptor type 2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download