Abstract
Nocardia species are aerobic, gram-positive pathogens found worldwide in soil. Nocardia is considered an opportunistic pathogen, and its infection mostly occurs in immunocompromised patients. We report a case of Nocardia farcinica induced mediastinitis and pneumonia that occurred in a 64-year-old male patient who had no significant medical history except for hypertension. He visited another hospital with a complaint of dyspnea and left chest wall pain. The symptoms arose 7 days ago without any trauma and they worsened. A mediastinal mass was found on computed tomography scan. After being transferred to our hospital for further evaluation, he was diagnosed with mediastinitis and pneumonia. As N. farcinica was found to be the causative organism by 16S rRNA sequencing, proper antibiotic therapy including trimethoprim/sulfamethoxazole was initiated immediately. After this, the patient improved and he was discharged. If an infection has a disseminating course, nocardiosis cannot be excluded even in immunocompetent patients. Once the diagnosis is established, prompt antibiotic therapy should be performed based on the severity.
Nocardia is soil-dwelling, gram-positive aerobic actinomycete causing a localized or disseminated infection1. The typical portal of entry of nocardiosis is the respiratory tract with subsequent dissemination to distant organs123.
Nocardia spp. are opportunistic pathogens that commonly infect patients with underlying immune compromise, pulmonary disease, or a history of surgery or trauma. Here, we report a case of N. farcinica mediastinitis and pneumonia in an immune-competent patient.
A 64-year-old male presented with 1-week history of left chest wall pain. The patient has no significant medical history except hypertension. When chest pain was aggravated and dyspnea was gradually developing, the patient visited another hospital. When the cardiac mass was found on radiologic study, the patient was transferred to our hospital for further evaluation. He was a farmer living in the countryside.
Initial vital signs were as follows: blood pressure, 130/70 mm Hg; heart rate, 78; respiration per minute, 24; and temperature, 36.7℃. Physical examinations revealed normal breathing sounds and heart sounds. Left pleural effusion was seen on simple chest X-ray and electrocardiogram showed normal findings. On arterial blood gas analysis, pH 7.450, pCO2 34 mm Hg, pO2 64 mm Hg, HCO3 23.6 mEq/L, SaO2 93% was noted. Leukocytosis (white blood cells 20,310/mm3, hemoglobin 12.2 g/dL, and platelets 583,000/mm3) was found in peripheral blood tests, but creatinine (0.68 mg/dL), blood urea nitrogen (8 mg/dL), total bilirubin (0.27 mg/dL), aspartate transaminase/alanine transaminase (19/29 IU/L) were within the normal range. C-reactive protein (CRP) was mildly elevated (2.49 mg/dL), but cardiac enzymes were normal.
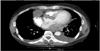
On chest computed tomography (CT), a 40-mm-sized low density mass (Figure 1) surrounded by necrotizing tissues was found in right pericardium with bilateral pleural effusions. Additional coronary CT excluded the possibility of acute coronary syndrome. The patient was admitted to our hospital under the impression of mediastinal mass or mediastinitis.
On next day of admission, body temperature raised to 38.2℃, dyspnea worsened. Increased pleural effusion was seen on chest X-ray. After intubation, effusions were drained percutaneously. To identify mediastinal mass visible on chest CT, we performed video-assisted thoracic surgery. Two mediastinal masses were found on operational findings. Left one was a 4-cm-sized, pus-containing mass liked to mediastinum and right mass was a smaller cystic lesion (Figure 2). Under impression of acute or chronic mediastinitis, the patient was treated with intravenous ceftriaxone and metronidazole after cultures were taken from blood, sputum and pleural fluid. On the sixth day of admission, the patient still had fever and left pleural effusion relapsed on X-ray. Laboratory evaluation revealed CRP increase to 17 mg/dL. A follow-up chest CT showed residual lesions on same sites. We repeated cultures since no bacterial growth identified from previous studies. Antibiotics were replaced by intravenous doripenem.
Five days later, fever still remained but chest X-ray showed improved effusions and CRP gradually decreased on laboratories. Moreover, blood cultures were still negative, but yellowish colonies with crimped surface were found in pleural fluid cultures. Therefore, we checked 16S rRNA sequencing under impression of nocardial infection. Compared to Macrogen Sequencing Database, 99% of rRNA were matched to N. farcinica. After that, we initiated intravenous prepenem with oral trimethoprim/sulfamethoxazole. After 2 weeks, patient was so improved that extubation was possible. One month later, we transferred the patient to another hospital with oral trimethoprim/sulfamethoxazole and amoxicillin/clavulanate.
Nocardia is a genus of aerobic actinomycetes responsible for localized or disseminated infections3. Nocardia spp. are aerobic, branching filamentous gram-positive, weakly acid-fast bacteria that live as soil saprophytes. There are more than thirty Nocardia species until now; thereof one-half are recognized as pathogens in human45. Though most common pathogen is N. asteroids, an infection with N. farcinica is potentially lethal because of its tendency to disseminated and its resistance to antibiotics1. Nocardiosis usually occurs in patients who has either impaired local pulmonary defenses or systemic immunosuppresion12 but rarely in healthy patients seen in this case.
The most common presentation of nocardiosis is pneumonia (75%) with fever, chills, dyspnea, and a productive cough6. Even when the clinical suspicion is high, the disease is commonly confused with other chronic suppurative lung diseases and malignancy because its pathogenesis is often subacute or chronic granulomatous789. Nocardiosis can be diagnosed rapidly by examination of sputum, pleural fluid, bronchial lavage fluid or percutaneous lung aspirates with the Gram stain and a modified acid-fast stain. Also, as demonstrated here, biochemical tests including 16S rRNA sequencing analysis may be used for identification.
The most important treatment for respiratory nocardiosis is appropriate antibiotics therapy in combination with surgical drainage in patients with brain abscesses or empyema10. A high percentage of Nocardia isolates are sensitive to sulfonamide and trimethoprim/sulfamethoxazole, but resistance is common among N. farcinica and N. otitidiscaviarum11. Nevertheless, the sulfonamides are still first-line agents for treat ment, as is the combination of trimethoprim/sulfamethoxazole12. In severely ill patients, carbapenem or amikacin can be used as part of combination therapy, considering the possibility of trimethoprim/sulfamethoxazole resistance13.
On previous studies, the overall mortality rate in patients with nocardiosis by N. farcinica is 14%, mostly caused by delayed diagnosis and inappropriate treatments. Especially, the mortality rate in patients with central nervous system involvement is 50%14, because its propensity for hematogenous dissemination, most commonly to the brain.
In conclusion, nocardiosis should be suspected when disseminating inflammations including brain abscess, pneumonia or cellulitis develop not only in immunocompromised patients, but even in immunocompetent individuals.
Figures and Tables
References
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996; 22:891–903.
2. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994; 7:357–417.
3. Yildiz O, Doganay M. Actinomycoses and nocardia pulmonary infections. Curr Opin Pulm Med. 2006; 12:228–234.
4. Han ST, Kim YS, Song SH, Uh Y, Han BG, Choi SO, et al. A case of nocardia farcinica brain abscess in a focal segmental glomerulosclerosis patient after steroid treatment. Korean J Nephrol. 2011; 30:98–101.
5. Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003; 41:4497–4501.
6. Uttamchandani RB, Daikos GL, Reyes RR, Fischl MA, Dickinson GM, Yamaguchi E, et al. Nocardiosis in 30 patients with advanced human immunodeficiency virus infection: clinical features and outcome. Clin Infect Dis. 1994; 18:348–353.
7. Apisarnthanarak A, Razavi B, Bailey T. Disseminated Nocardia asteroides presenting as pulmonary non-caseating granulomas in a patient with Waldenstrom macroglobulinemia. Infection. 2002; 30:38–40.
8. Russo TA. Agents of actinomycosis. In : Mandell GL, Bennet JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Philadelphia: Churchill Livingstone;2005. p. 2924–2934.
9. Bartlett JG. Agents of actinomycosi. In : Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. 2nd ed. Philadelphia: WB Saunders;1998. p. 1973–1980.
10. LoCicero J 3rd, Shaw JP, Lazzaro RS. Surgery for other pulmonary fungal infections, Actinomyces, and Nocardia. Thorac Surg Clin. 2012; 22:363–374.
11. Wallace RJ Jr, Septimus EJ, Williams TW Jr, Conklin RH, Satterwhite TK, Bushby MB, et al. Use of trimethoprim-sulfamethoxazole for treatment of infections due to Nocardia. Rev Infect Dis. 1982; 4:315–325.
12. Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore). 2004; 83:300–313.
13. Roberts SA, Franklin JC, Mijch A, Spelman D. Nocardia infection in heart-lung transplant recipients at Alfred Hospital, Melbourne, Australia, 1989-1998. Clin Infect Dis. 2000; 31:968–972.
14. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006; 19:259–282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download