Abstract
Ankylosing spondylitis is a chronic inflammatory multisystem disease that primarily affects the axial joints. Pleuropulmonary involvement is an uncommon extra-articular manifestation of ankylosing spondylitis. There is a wide spectrum of pulmonary parenchymal changes in ankylosing spondylitis, beginning in the early stages of the disease and increasing over time. The lesions are usually asymptomatic, and not visible on chest radiographs in early stages. We reported a case of advanced ankylosing spondylitis in a 56-year-old man with progressive pulmonary bullous fibrocystic changes on both upper lobes that were misdiagnosed as tuberculosis in the early stages of the disease.
Ankylosing spondylitis is a chronic inflammatory multisystem disease that affects the skeletal system and, uncommonly, an extra-skeletal system. Ankylosing spondylitis may present with inflammatory arthritis of the peripheral joints and can involve extra-articular structures such as the lungs, kidneys, eyes, aorta and the heart1. Respiratory abnormalities have been reported in up to 30% of patients with ankylosing spondylitis2. Chest wall rigidity may occur due to costovertebral joint involvement. Ankylosing spondylitis can cause pleuropulmonary lesions, most commonly associated with diseases such as apical fibrosis, interstitial infiltrates, and pleural thickening2. In a high-resolution computed tomography (HRCT) study, lung parenchymal changes and apical fibrosis were found in 59% and 10% of patients, respectively3. Pulmonary changes in ankylosing spondylitis have been evaluated by chest X-ray, pulmonary function tests, and bronchoalveolar lavage4. In the mid-1980s when HRCT was developed, accurate diagnosis of lung parenchymal lesions became possible4. Pleuropulmonary complications associated with ankylosing spondylitis are initially radiologically similar to early tuberculosis with apical lesions. They progress to fibrocystic changes accompanying bronchiectasis with or without cavities and are therefore usually misdiagnosed as sequelae of pulmonary tuberculosis5.
We recently encountered a case of advanced ankylosing spondylitis with progressive pulmonary fibrocystic bullous changes on both upper lobes.
A 56-year-old man who developed ankylosing spondylitis at the age of 33 in 1986 complained of dyspnea and back pain for 10 days before his first admission to the pulmonology department of our hospital via the emergency room in 2009. At another hospital in 2002, he had been treated with internal fixation of the thoracic and lumbar spine due to an ankylosing spondylitis-associated spine deformity. He was clinically diagnosed with smear-negative pulmonary tuberculosis in 2004 and received incomplete treatment. In 2007, he was diagnosed with reactivation of pulmonary tuberculosis and took anti-tuberculosis agents for 9 months . Medical records review showed that he was clinically diagnosed with smearnegative, culture-negative pulmonary tuberculosis based only on radiological findings and without mycobacterial confirmation. At the time of admission to out hospital, the patient was not taking drugs other than anti-tuberculous agents and nonsteroidal anti-inflammatory drugs for intermittent pain control. He has smoked half a pack of cigarettes daily for 15 years with occasional alcohol intake.
The patient was diagnosed with pneumonia and an exacerbation of chronic obstructive pulmonary disease at first admission. Therapy with daily intravenous antibiotics was begun following multiple blood, sputum , and urine cultures. Oral and inhaled bronchodilators were prescribed and oral prednisolone (30 mg) was maintained for 10 days. Since discharge in 2009, he has maintained medical treatments and respiratory rehabilitation. Treatment consists of oral medication, including methylxanthine, a long-acting beta 2 agonist (LABA), with inhaled therapy of a long-acting anticholinergic, inhaled corticosteroid and LABA combination.
The patient's initial chest radiograph, taken by the orthopedic department in 2006, showed apical linear fibrotic opacity with cystic changes similar to sequelae of pulmonary tuberculosis (Figure 1A). A nother chest radiograph, taken in the pulmonology department at first admission in 2009, showed patchy consolidations and ground-glass opacities in both lungs, and apical fibrosis with bullous changes suggesting stable tuberculosis and emphysema in both lungs (Figure 1B). Over the next 8 years, the patient's serial chest radiographs (Figure 1C) and HRCT findings (Figure 2) showed bullous fibrocystic changes on both upper lobes, and the bullous cysts increased in size with disease duration. HRCT findings (Figure 2) revealed linear fibrocystic opacity, calcified granulomas, and traction bronchiectasis accompanying parenchymal lung destruction in both upper lungs compatible with post-tuberculosis sequelae, and pneumonic consolidations wi th ground-glass opacities in both lower lobes.
During a follow-up period of 6 years from 2009 to 2014 in the pulmonology department of our hospital, the patient experienced frequent respiratory events and was hospitalized ten times. Among his respiratory events, pneumonia was the most common reason requiring a visit to the hospital, followed by acute exacerbation of chronic obstructive pulmonary disease and hemoptysis. The patient was hospitalized via the emergency room due to massive hemoptysis following superinfection of the cavitary-destroyed lung with mycetoma, and underwent bronchial artery embolization in combination with antifungal agents at the age of 60 in 2013. The pathogen was not confirmed by sputum or blood culture, but since aspergillus antigen was confirmed positive in serum, the lung lesions were thought to be associated with a fungal infection. Chest radiography showed that a fungal ball had been present in a fibrocavitary lesion of the left upper lung since 2011. The patient complained of symptoms such as worsening dyspnea and required oxygen therapy at home because of chronic respiratory failure.
In analyses of radiologic serial images with chest X-ray and HRCT, the progression of parenchymal lung destruction was correlated with disease duration in this patient with advanced ankylosing spondylitis. Chest X-ray (Figure 1) and HRCT (Figure 2) showed that the initial apical fibrosis in 2009 progressed to progressive fibrocystic bullous changes that involved both upper lobes with cavitations. In serial films, frequent superinfections of cavitary lung lesion with mycetoma in both upper lungs were noted. Although the patient was clinically diagnosed with smear-negative pulmonary tuberculosis and reactivation during previous treatment at another hospital, acid-fast staining and all subsequent mycobacterial sputum cultures were negative, and an interferon-γ release assay failed to detect mycobacterial infection.
Following the investigation and retrospective review of medical records and radiologic findings by a pulmonologist, a rheumatologist, and a chest radiologist, the patient's progressive fibrocystic bullous lung changes were interpreted as pleuropulmonary involvement of extra-articular symptoms of advanced ankylosing spondylitis. The patient is currently managed at home with supportive care, maintenance medical treatment, and oxygen therapy. Considering the generally poor performance of patients with advanced-stage disease and the high risk of complications related to active treatments, surgical resection procedures, such as bullectomy, and anti-tumor necrosis factor α (TNF-α) agents are not being used.
To our knowledge, the case described her e is the first in Korea in which progressive pulmonary fibrobullous cystic changes were diagnosed as advanced ankylosing spondylitis-associated pleuropulmonary involvement with confirmation of long-term progression. Although previous reports of pulmonary fibrobullous cystic changes associated with ankylosing spondylitis exist, this case shows fibrobullous lung lesions in both upper lobes without significant interval changes6.
Pleuropulmonary abnormalities in ankylosing spondylitis are usually evaluated using chest X-ray, pulmonary function test, and bronchoalveolar lavage7. However, due to recent diagnostic advances such as HRCT, highly sensitive examinations of the entire lung parenchyma and pleura are now possible4. HRCT findings are characterized by apical fibrosis, parenchymal bands, septal thickening, emphysema, pleural thickening, upper lobe fibrobullous disease, interstitial lung disease, and pleural effusion3. According to several previous reports, pleural and pulmonary involvement of ankylosing spondylitis are present in up to 30% of patients6. Even if ankylosing spondylitis involves the lung, early-stage patients are asymptomatic in most cases3. Pulmonary abnormalities are typically identified about 11.7 years after the disease occurs8.
In many cases, pulmonary fibrocystic cavitary changes due to ankylosing spondylitis are similar to signs of pulmonary tuberculosis in radiologic studies9. Diagnostic tests, such as sputum acid-fast bacilli staining with cultures, nucleic-acid amplification tests for tuberculosis, and interferon-γ release assays, are usually performed when tuberculosis is suspected. However, in most cases, it is difficult to confirm Mycobacterium tuberculosis6. Pulmonary tuberculosis has been diagnosed in a few ankylosing spondylitis patients10, but reports suggest they do not show improvement after treatment6. Therefore, we suggest that extra-articular symptoms in patients with ankylosing spondylitis may be misdiagnosed as tuberculosis3. It is difficult to distinguish the lung lesions of ankylosing spondylitis from old tuberculosis or sequelae of other infection, such as pneumonia, by X-ray. If the symmetric fibrosis of both upper lung zones shows progression over time and does not occur after infection, the lung lesions can be considered as being ankylosing spondylitis-associated. In this case, we have performed long-term follow-up chest X-rays and HRCT since 2006. The patient was treated with anti-tuberculous agents twice in the past for lung lesions detected on radiologic findings without bacteriologic confirmation, but fibrobullous changes in both upper lungs worsened as disease severity and duration increased. Therefore, the patient was diagnosed with pleuropulmonary involvement of ankylosing spondylitis.
Apical fibrobullous areas are often targets of aspergillus infection11. Chronic aspergillus colonization is identified in 50% to 65% of patients with ankylosing spondyloarthritis12. Hemoptysis is the most common symptom in patients with aspergilloma13, and the treatment of choice for symptomatic aspergilloma is antifungal agents9. When medical treatment is insufficient, thoracic surgery may be indicated12. However, in our case, the patient did not undergo thoracic surgery because hemoptysis improved with medical treatment and thoracic surgery held high risks.
There are two main theories regarding the mechanisms that cause lung fibrosis in ankylosing spondylitis2. One of the two theories is a mechanical theory. Chest wall rigidity may occur due to hyperventilation of the lung base and hypoventilation of the lung apices. The poor airway clearance caused by apical hypoventilation may induce chronic inflammation. The second theory is a disease-specific theory. It has been confirmed that there is no difference in mucociliary function between the upper lung and lower lung14. This result suggests that apical fibrosis is caused by a specific mechanism related to the nature of ankylosing spondylitis2. Tobacco smoke worsens the lung disease by increasing macrophages and neutrophils in the lung parenchyma2.
There is no effective treatment to stop progression of pulmonary involvement in patients with ankylosing spondylitis, although smoking cessation and respiratory rehabilitation may improve overall lung function2. The therapeutic effects of anti-TNF-α on lung fibrosis have not been evaluated1. However, these agents are widely prescribed for patients with ankylosing spondylitis, and case reports describe beneficial effects in patients with interstitial lung disease associated with rheumatoid arthritis. Therefore, anti-TNF-α agents have been considered as a treatment option for these patients1. However, there is a risk of reactivation of latent tuberculosis, and therefore proper monitoring and evaluation should accompany treatment. In addition, during ankylosing spondylitis treatment with anti-inflammatory medications, such as infliximab, etanercept, and adalimumab, improvements in pulmonary manifestations may not be clear15.
This case report shows that progressive pulmonary fibrobullous cystic changes are associated with advanced ankylosing spondylitis and correlate with disease duration. In the early stages, pleuropulmonary involvement may be misdiagnosed as tuberculosis. Therefore, diagnostic studies should be performed for bacteriologic confirmation and long-term serial radiologic follow-up. Further comprehensive studies are necessary to evaluate the effectiveness of treatments to delay or stop pleuropulmonary involvement in patients with ankylosing spondylitis.
Figures and Tables
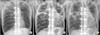 | Figure 1Bullous fibrocystic changes on serial chest X-rays taken in 2006 (A), 2009 (B), and 2014 (C). (A) Initial chest radiograph showing apical linear fibrotic opacities with cystic changes in both upper lobes. In addition, is internal fixation of the thoracolumbar spine for the treatment of spinal deformity due to ankylosing spondylitis, is observed. (B) Follow-up chest radiograph taken at the first admission due to pneumonia demonstrates consolidation in both lungs and marked progression of apical fibrobullous changes. (C) Last follow-up chest radiograph reveals increased size of bullous cysts in both upper lobes. Patchy consolidations are also noted in both lower lungs, suggestive of superinfection. |
 | Figure 2Bullous fibrocystic changes in serial chest high-resolution computed tomography (HRCT) taken in 2009 (A) and 2014 (B). (A) HRCT showing linear fibrotic opacities and bullae in both lungs. Combined consolidations in both lungs and small pleural effusion are suggestive of combined infection. (B) Follow-up HRCT reveals progression of fibrobullous lesions with increasing bullae size. |
References
1. Quismorio FP Jr. Pulmonary involvement in ankylosing spondylitis. Curr Opin Pulm Med. 2006; 12:342–345.
2. El Maghraoui A. Pleuropulmonary involvement in ankylosing spondylitis. Joint Bone Spine. 2005; 72:496–502.
3. Hasiloglu ZI, Havan N, Rezvani A, Sariyildiz MA, Erdemli HE, Karacan I. Lung parenchymal changes in patients with ankylosing spondylitis. World J Radiol. 2012; 4:215–219.
4. Kiris A, Ozgocmen S, Kocakoc E, Ardicoglu O, Ogur E. Lung findings on high resolution CT in early ankylosing spondylitis. Eur J Radiol. 2003; 47:71–76.
5. Jessamine AG. Upper lung lobe fibrosis in ankylosing spondylitis. Can Med Assoc J. 1968; 98:25–29.
6. Kim YS, Na UK, Chung YT, Park CS. A case of ankylosing spondylitis associated with fibrobullous lung lesion. Tuberc Respir Dis. 1988; 35:138–143.
7. Feltelius N, Hedenstrom H, Hillerdal G, Hallgren R. Pulmonary involvement in ankylosing spondylitis. Ann Rheum Dis. 1986; 45:736–740.
8. El Maghraoui A, Dehhaoui M. Prevalence and characteristics of lung involvement on high resolution computed tomography in patients with ankylosing spondylitis: a systematic review. Pulm Med. 2012; 2012:965956.
9. Pamuk ON, Harmandar O, Tosun B, Yoruk Y, Cakir N. A patient with ankylosing spondylitis who presented with chronic necrotising aspergillosis: report on one case and review of the literature. Clin Rheumatol. 2005; 24:415–419.
10. Davies D. Ankylosing spondylitis and lung fibrosis. Q J Med. 1972; 41:395–417.
11. Thai D, Ratani RS, Salama S, Steiner RM. Upper lobe fibrocavitary disease in a patient with back pain and stiffness. Chest. 2000; 118:1814–1816.
12. Pontier S, Bigay L, Doussau S, Recco P, Lacassagne L, Didier A. Chronic necrotizing pulmonary aspergillosis and ankylosing spondylarthritis. Rev Mal Respir. 2000; 17:683–686.
13. Miller WT. Aspergillosis: a disease with many faces. Semin Roentgenol. 1996; 31:52–66.
14. Bouvier M, Guyonnet G, Tebib J, Massonnet B, Azzar D. Apical fibrosis in ankylosing spondylitis: contribution of the mucociliary clearance. Presse Med. 1988; 17:1493–1494.
15. Kanathur N, Lee-Chiong T. Pulmonary manifestations of ankylosing spondylitis. Clin Chest Med. 2010; 31:547–554.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download