Abstract
Excessive dynamic airway collapse (EDAC) is a disease entity of excessive reduction of the central airway diameter during exhalation, without cartilage collapse. An 80-year-old female presented with generalized edema and dyspnea at our hospital. The patient was in a state of acute decompensated heart failure due to pneumonia with respiratory failure. We accordingly managed the patient with renal replacement therapy, mechanical ventilation and antibiotics. Bronchoscopy confirmed the diagnosis of EDAC. We scheduled extubation after the improvement of pneumonia and heart condition. However, extubation failure occurred due to hypercapnic respiratory failure with poor expectoration. Her EDAC was improved in response to high flow nasal oxygen therapy (HFNOT). Subsequently, the patient was stabilized and transferred to the general ward. HFNOT, which generates physiologic positive end expiratory pressure (PEEP) effects, could be an alternative and effective management of EDAC. Further research and clinical trials are needed to demonstrate the therapeutic effect of HFNOT on EDAC.
Excessive dynamic airway collapse (EDAC) is defined as more than 50% reduction of the sagittal diameter of the trachea in forced expiration or during coughing. It is characterized by an excessive collapse of the posterior membranous trachea towards the lumen without cartilage collapse1234. EDAC presents with a variety of symptoms ranging from mild shortness of breath or cough to respiratory failure56. Advances in imaging modalities, including bronchoscopy and dynamic radiographic studies, allow increased recognition of EDAC7. Current therapeutic management of EDAC includes conservative medical therapy, including bronchodilators and continuous positive airway therapy, and minimally invasive and open surgical interventions8.
Recently, there has been growing interest in high flow nasal oxygen therapy (HFNOT). HFNOT has been known to generate physiological positive airway pressure effect. Therefore, it was suggested that HFNOT could be replace to continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV)9. However, there was no study about HFNOT was used in management of EDAC.
Herein, we report a case of EDAC that caused inability to wean from mechanical ventilation, and which was successfully managed with HFNOT.
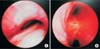
An 80-year-old female came to hospital because of general weakness for 1 week. She had diabetes mellitus and diastolic heart failure previously. She had symptoms like cough, increased sputum and 14 kg weight gain for 1 month (from 48 to 62 kg). At initial, her saturation (SpO2) was 78% in room air. There were wheezing and diffuse crackle sound in both whole lung fields. Multifocal patchy consolidations in right upper lobe and both lower lobe were presented in her chest anteroposterior and chest computed tomography (CT) (Figure 1). White blood cell (WBC) count was 7,900/µL, blood urea nitrogen/serum creatinine 48/1.83 mg/dL, FeNa 1.28%, N-terminal of the prohormone brain natriuretic peptide 6,748 pg/mL, urine WBC ≥100/high-power field (HPF), urine bacteria 2+/HPF were checked. We assessed the patients with acute decompensated heart failure due to respiratory infection and acute kidney injury with urinary tract infection. Despite diuretics, oxygen therapy, empiric antibiotics and bronchodilator therapy, her hypoxemia with hypercapnea was aggravated. Eventually, she was intubated and mechanically ventilated in hospital day (HD) 1. For microbiologic evaluation of purulent sputum, we performed diagnostic bronchoscopy in HD 3, coincidently we found out excessive dynamic airway compression in exhalation and purulent secretion in her bronchoscopy (Figure 2). Oxygen requirement of the patients was reduced in according to pneumonia was improved, so we tried to wean from mechanical ventilation in HD 13. However, carbon dioxide (CO2) retention and poor expectoration of secretion cause failure to wean. Follow-up bronchoscopy was done for evaluating airway status, so we found EDAC was sustained (Figure 3). Therefore, we performed percutaneous dilatational tracheostomy and tried HFNOT with FiO2 0.5 flow 30 L/min in HD 14. We found that the excessive dynamic tracheal compression in exhalation was decreased after applying of HFNOT (video file was showed in supplementary material, Video clip 1) (Figure 4), and CO2 retention and expectoration were improved. After 3 days, she was stable, so she was transferred to general ward with HFNOT.
EDAC and tracheobronchomalacia (TBM) are two different disease entities, included in expiratory central airway collapse. EDAC is a disease characterized by excessive bulging of the posterior membrane into the airway lumen during exhalation. On the other hand, there is weakness of the tracheobronchial cartilaginous walls in TBM1234. The reported prevalence of TBM and EDAC varies, reporting 4%-23% in patients undergoing bronchoscopy310, and EDAC was present in 22% in chronic obstructive pulmonary disease (COPD) and/or asthma patients4.
These diseases are probably underdiagnosed because it shows nonspecific symptoms similar to patients with obstructive lung disease like COPD and asthma8. They can cause chronic cough, dyspnea, difficulty clearing secretions, recurrent bronchitis or pneumonia, syncope during episodes of coughing, pulmonary edema, and respiratory failure5611. In the case of respiratory failure, the patient may experience difficulties to wean or unexplained extubation failure12. Physical examination may reveal wheezing, rhonchi, and decreased breath sounds6.
EDAC can be diagnosed using dynamic bronchoscopy or dynamic radiologic imaging such as dynamic CT. Suggestive symptoms and pulmonary function test help to diagnosis of EDAC, but they have no diagnostic value and is not predictive of disease progress. Otherwise, we should know that EDAC diagnosis is difficult because of bronchoscopy or CT performed under mechanical ventilation could give negative results2.
Treatment for EDAC depends on the severity of symptoms, degree, and extent of airway collapse. Many patients with EDAC are asymptomatic, therefore they should not be treated8. Proposed management strategies include conservative therapy (bronchodilators, CPAP, and NIPPV), minimally invasive therapy (endoluminal airway stents and laser therapy), surgical therapy (tracheostomy, airway splinting, and tracheal resection)8. Among them, CPAP and NIPPV act as pneumatic stents. Positive pressure application was known to decrease pulmonary resistance and improve airflow in patients with expiratory airflow obstruction13.
In this case, we diagnosed EDAC in patients by bronchoscopy. We could not perform pulmonary function test because of the patient was bed-ridden state in hospital. We could guess that EDAC of the patients contributed to retention of CO2 and poor expectoration, therefore it caused failure to wean from mechanical ventilation. We tried HFNOT for applying intermittent positive end expiratory pressure (PEEP) in this case, and it worked well. To our knowledge, this case is the first case about HFNOT applying in management of EDAC. HFNOT is known to have a number of physiological effects. In previous several studies, HFNOT generates a low level of PEEP14, improves oxygenation, increased the end-inspiratory lung volume, reduces airway resistance, increased functional residual capacity14, and makes better mucociliary clearance15. Therefore, we recommend that HFNOT can be an effective management method in EDAC.
There are no randomized studies about HFNOT applying in EDAC. Therefore, further research and clinical trials are needed to demonstrate the therapeutic effect of HFNOT on EDAC.
Figures and Tables
Figure 1
The initial chest radiography at our hospital shows patchy consolidations in left lower lung field and right upper lung field.

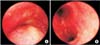
Figure 2
The initial bronchoscopy shows excessive dynamic airway compression at exhalation (A) and inhalation (B).
References
1. Murgu SD, Colt HG. Description of a multidimensional classification system for patients with expiratory central airway collapse. Respirology. 2007; 12:543–550.
2. Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006; 11:388–406.
3. Kalra A, Abouzgheib W, Gajera M, Palaniswamy C, Puri N, Dellinger RP. Excessive dynamic airway collapse for the internist: new nomenclature or different entity? Postgrad Med J. 2011; 87:482–486.
4. Park JG, Edell ES. Dynamic airway collapse: different from tracheomalacia. Rev Port Pneumol. 2005; 11:600–602.
5. Murgu SD, Cherrison LJ, Colt HG. Respiratory failure due to expiratory central airway collapse. Respir Care. 2007; 52:752–754.
6. Nuutinen J. Acquired tracheobronchomalacia: a clinical study with bronchological correlations. Ann Clin Res. 1977; 9:350–355.
7. Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse: novel diagnostic tools clarify the issues. Pulm Perspect. 2005; 22:7–10.
8. Murgu SD, Colt HG. Treatment of adult tracheobronchomalacia and excessive dynamic airway collapse: an update. Treat Respir Med. 2006; 5:103–115.
9. Gotera C, Diaz Lobato S, Pinto T, Winck JC. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol. 2013; 19:217–227.
10. Ikeda S, Hanawa T, Konishi T, Adachi M, Sawai S, Chiba W, et al. Diagnosis, incidence, clinicopathology and surgical treatment of acquired tracheobronchomalacia. Nihon Kyobu Shikkan Gakkai Zasshi. 1992; 30:1028–1035.
11. Firdose R, Elamin E. Pulmonary edema secondary to dynamic tracheal collapse. J Bronchol. 2004; 11:118–121.
12. Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an indepth review. Chest. 2005; 127:984–1005.
13. Martin JG, Shore S, Engel LA. Effect of continuous positive airway pressure on respiratory mechanics and pattern of breathing in induced asthma. Am Rev Respir Dis. 1982; 126:812–817.
14. Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011; 37:1780–1786.
15. Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011; 56:1151–1155.
Supplementary Material
Supplementary material can be found in the journal homepage (http://www.e-trd.org).
Video clip 1
The bronchoscopic video of case. While the sagittal diameter of the trachea collapses about 90% in initial 15 seconds, after high flow nasal oxygen therapy (HFNOT) applying, the diameter of the trachea was recovered. Then, the diameter of the trachea re-collapses after taking off the HFNOT in the last 15 seconds.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download