Abstract
Small cell lung cancer (SCLC), which originated from neuroendocrine tissue, can develop into paraneoplastic endocrine syndromes, such as Cushing syndrome, because of an inappropriate secretion of ectopic adrenocorticotropic hormone (ACTH). This paraneoplastic syndrome is known to be a poor prognostic factor in SCLC. The reason for poor survival may be because of a higher risk of infection associated with hypercortisolemia. Therefore, early detection and appropriate treatment for this syndrome is necessary. But the diagnosis is challenging and the source of ACTH production can be difficult to identify. We report a 69-year-old male patient who had severe hypokalemia, metabolic alkalosis, and hypertension as manifestations of an ACTH-secreting small cell carcinoma of the lung. He was treated with ketoconazole and spironolactone to control the ACTH dependent Cushing syndrome. He survived for 15 months after chemotherapy, which is unusual considering the poor outcome of the ectopic ATH syndrome associated with SCLC.
Small cell lung cancer (SCLC) develops from neuroendocrine cells and expresses various peptides and protein factors which can play as hormones. Although syndrome of inappropriate secretion of antidiuretic hormone and Cushing syndrome due to ectopic adrenocorticotropic hormone (ACTH) secretion are the most common paraneoplastic syndromes in SCLC, the latter occurs in only 1%-5% of SCLC1. Ectopic ACTH can be secreted from original cancer tissues or metastatic lesions of SCLC. These patients generally present with electrolyte disturbances rather than typical cushingoid feature. Typical cushingoid features such as moon face or buffalo hump do not usually appear because the hypercortisolism is an acute phenomenon and the patients generally do not survive long enough until morphologic changes occur2. It is the reason why patients with Cushing syndrome due to ectopic ACTH syndrome (EAS) are easily overlooked. Therefore, it is necessary to suspect the disease at the initial view to improve the poor clinical outcome.
We report a case of SCLC with Cushing syndrome due to EAS from the original tumor tissue in a 69-year-old male presenting with severe hypokalemia, metabolic alkalosis, and hypertension without any respiratory symptoms. The clinical features and optimal management are discussed.
A 69-year-old male with underlying hypertension was admitted with a complaint of general weakness started 10 days ago. The patient had been taking irbesartan 150 mg and thiazide 12.5 mg for 3 years. He had 20 pack-year history of smoking. The initial blood pressure was 128/82 mm Hg, the respiratory rate 20 breaths per minute, and the temperature was 36℃. Chest auscultation revealed decreased breathing sound on the left upper area of chest wall.
Laboratory findings were as follows: white blood cell count, 8,190×109/L; hemoglobin, 16.4 g/dL; platelet count, 170×109/L; glucose level, 235 mg/dL; sodium, 141 mEq/L; potassium, 1.7 mEq/L; chloride, 88 mEq/L; lactic dehydrogenase, 863 U/L; D-dimer, 0.08 µg/L; and C-reactive peptide, 2.30 mg/L. Arterial blood gas analysis was pH 7.574, pCO2 50.1 mm Hg, pO2 122 mmHg, HCO3 40 mmol/L, and oxygen saturation was 98.8% suggesting metabolic alkalosis.
The chest radiography taken on admission showed left upper lobe atelectasis and mass like lesion infiltrating the left upper bronchus (Figure 1). Chest computed tomography (CT) scans revealed about 7×5-cm-sized mass in left upper lobe showing invasion to the main pulmonary arterial trunk (Figure 2). Biopsy was done with bronchoscopy and the pathology confirmed small cell carcinoma. Positron emission tomography CT showed hepatic and both adrenal gland metastases.
Our primary impression was hyperaldosteronism or pseudohyperaldosteronism according to the lab finding of hypokalemia associated with hypertension. Aldosterone and renin level being normal, hypokalemia with metabolic alkalosis in patient with lung cancer infiltrating left upper lobe suggested Cushing syndrome due to ectopic ACTH secreting tumor. The ACTH level was elevated to 535.61 pg/mL and the cortisol level was elevated to 47.43 µg/dL. The level of cortisol was not suppressed with low dose and high dose dexamethasone suppression test and the 24-hour urinary free cortisol was still elevated to 1,796.93 µg/day. Brain magnetic resonance imaging showed normal pituitary gland and no cerebral metastases. The malignant tissue obtained with bronchoscopy was positive in ACTH marker stain. These finding were consistent with Cushing syndrome caused by ectopic ACTH production (Figure 3). The patient had no cushingoid appearance such as moon face, skin change or central obesity, and hypokalemia was the only clinical feature to suspect diagnosis.
To reduce the high cortisol level before chemotherapy, ketoconazole (400 mg two times a day) and spironolactone were administered for EAS. As hypokalemia resolved, so did the metabolic alkalosis. It was not needed to maintain corticosteroid treatment after normalization of hypokalemia. Once ectopic ACTH production controlled, the patient was transferred to the oncology division to have systemic chemotherapy and survived for 15 months.
Ectopic ACTH production is defined as the secretion of ACTH from a tumor outside the pituitary gland. Cushing syndrome due to EAS was first described in 1965 and it causes 5%-10% of cases of spontaneous Cushing syndrome34. SCLC and bronchial carcinoids account for about half of the cases but it can appear in various tumors like pancreas cancer, medullary thyroid cancer, thymoma and pheochromocytoma. Clinical Cushing syndrome secondary to ectopic ACTH production is uncommon, occurring in 3.2%-4.5% of SCLC patients56. The patients generally present with electrolyte disturbances rather than typical cushingoid feature. As our case showed, hypokalemia is the usual finding that suggests the presence of EAS and lead to subsequent confirmation test5.
Generally, patients with ectopic ACTH production have high ACTH levels (>20 ng/L), cortisol levels which is not suppressed with high doses of dexamethasone (8 mg/day) and demonstrate negative responses of pituitary gland to croticotropin-releasing hormone. However some patients with ectopic ACTH production demonstrate suppressed cortisol level on high-dose of dexamethasone. In that case the most useful test for differential diagnosis is the inferior petrosal sinus sampling7.
When the primary ACTH secreting tumor is found, the option of medical treatments is to administrate glucocorticoid synthesis inhibitors such as ketoconazole, mitotane, metyrapone, and octreotide8. Other medical interventions include potassium replacement and spironolactone. Ketoconazole which blocks corticosteroid production by inhibiting 17-hydroxylase and 11-hydroxylase, is the therapy of choice because of its low incidence of side effects9.
There are some evidences that mortality is increased in patients who have Cushing syndrome associated with EAS1011. Retrospective studies have shown that median survival of SCLC patients with Cushing syndrome ranges from 3.5 to 5.5 months, suggesting that the presence of EAS is a poor prognostic factor12. Marked suppression of the immune system by high cortisol level may cause severe infections, which easily can lead to septicemia and opportunistic infection9. The appearances of hypokalemia, metabolic alkalosis, and hypertension shown in these patients also seemed to be the result of sustained activation of circulating cortisol. It is thought that lowering cortisol levels before attempting curative treatments such as surgery or chemotherapy, may reduce the mortality and morbidity associated with Cushing syndrome13. However, the diagnosis is challenging, because the source of ACTH production can be difficult to be identified. Early detection and appropriate treatment for this syndrome are so difficult that survival remains low for this group of patients.
In Korea, few cases were reported about EAS associated with SCLC and the overall survival was within 2 months up to now. In our case, early diagnosis of EAS was made, so the patient received proper management to control the high level of cortisol and could have systemic chemotherapy thereafter. He survived for 15 months after chemotherapy, which is not common considering the poor outcome of EAS associated with SCLC.
As our case showed, it is necessary to suspect the disease at the initial view to improve the poor clinical outcome. The possibility of hypercortisolism caused by malignancy should be considered in patient presenting with hypertension, diabetes, hypokalemia, and metabolic alkalosis with high level of cortisol. In conclusion, EAS associated with SCLC has been known to have poor prognosis with low response to chemotherapy. The detection of EAS is difficult because of absence of typical features and low incidence. As our case showed, early suspicion of EAS and prompt management are clinically important to improve poor outcomes in patients with SCLC.
Figures and Tables
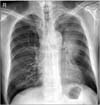 | Figure 1Chest radiography showed an ill-defined mass at the left hilum suggesting a mass involving the left upper bronchus with marked atelectasis of the left upper lobe. |
Acknowledgements
We would like to thank the patient whose written consent was obtained for publication of his medical history within this case report.
References
1. Mayer S, Cypess AM, Kocher ON, Berman SM, Huberman MS, Hartzband PI, et al. Uncommon presentations of some common malignancies: Case 1. Sequential paraneoplastic endocrine syndromes in small-cell lung cancer. J Clin Oncol. 2005; 23:1312–1314.
2. Howlett TA, Rees LH, Besser GM. Cushing's syndrome. Clin Endocrinol Metab. 1985; 14:911–945.
3. Liddle GW, Givens JR, Nicholson WE, Island DP. The ectopic ACTH syndrome. Cancer Res. 1965; 25:1057–1061.
4. Nieman LK. Cushing's syndrome. Curr Ther Endocrinol Metab. 1997; 6:161–164.
5. Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol. 1992; 10:21–27.
6. Abeloff MD, Trump DL, Baylin SB. Ectopic adrenocorticotrophic (ACTH) syndrome and small cell carcinoma of the lung-assessment of clinical implications in patients on combination chemotherapy. Cancer. 1981; 48:1082–1087.
7. Kenchaiah M, Hyer S. Cushing's syndrome due to ectopic ACTH from bronchial carcinoid: a case report and review. Case Rep Endocrinol. 2012; 2012:215038.
8. Yang HJ, Sung HJ, Kim JE, Lee HJ, Park JM, Park CK, et al. A case of ectopic ACTH syndrome associated with small cell lung cancer presented with hypokalemia. J Korean Endocr Soc. 2007; 22:359–364.
9. Izzedine H, Besse B, Lazareth A, Bourry EF, Soria JC. Hypokalemia, metabolic alkalosis, and hypertension in a lung cancer patient. Kidney Int. 2009; 76:115–120.
10. Dimopoulos MA, Fernandez JF, Samaan NA, Holoye PY, Vassilopoulou-Sellin R. Paraneoplastic Cushing's syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer. 1992; 69:66–71.
11. Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000; 85:42–47.
12. Lokich JJ. The frequency and clinical biology of the ectopic hormone syndromes of small cell carcinoma. Cancer. 1982; 50:2111–2114.
13. Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011; 117:4381–4389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download