Abstract
Fat embolism syndrome (FES) is a clinical manifestation that consists of multiple organ dysfunction due to fat emboli. FES occurs as a complication after trauma or procedures such as surgery. The diagnostic criteria of FES have not yet been established, so clinical criteria are used for its diagnosis. The clinical course of acute fulminant FES can be rapid. Liposuction surgery, in which adipocytes are mechanically disrupted, is one cause of FES. As the number of liposuction surgeries increases, clinicians should be aware of the possibility of FES. This was the first report of a case of acute fulminant FES with severe acute respiratory distress syndrome after liposuction surgery, in Korea.
Fat embolism syndrome (FES) is a clinical manifestation that consists of multiple organ dysfunction (involving the lungs, brain, and cardiovascular system) caused by fat emboli1. FES is mainly seen after procedures or conditions such as orthopedic surgery, severe burns, liver injury, and liposuction1. Although the precise mechanisms of FES remain unclear, intravasation of fat or fatty acids from long bone fractures and other sources is considered the primary cause, and the presence of these materials within the systemic circulation leads to multiple organ failure234.
Many cases of FES with lung involvement after bone fracture have been described in Korea. However, until now, no cases of FES after liposuction surgery with multiple organ failure have been reported in Korea. Here we present a case of a 21-year-old Asian man who experienced FES with acute respiratory failure, cerebral dysfunction, and acute heart failure after liposuction surgery.
A 21-year-old Asian man visited our hospital with fever, dyspnea, and hemoptysis. He was healthy except for obesity and he was college student (music major).
The patient was referred to our hospital while intubated and on oxygen. He underwent elective liposuction surgery under general anesthesia at a local medical center 8 hours before his admission. His body mass index was 32.5 kg/m2. A preoperative evaluation showed no abnormalities and the intraoperative period was uneventful. However, 1 hour after extubation, he became breathless and the hypoxia worsened to oxygen saturation of 50%. Hemoptysis was observed and fever developed. He showed impaired mental state and was transferred to the emergency center of an adjacent hospital. He was immediately intubated and required mechanical ventilation. After chest computed tomography (CT) was performed, he was referred back to our hospital with suspected acute respiratory distress syndrome (ARDS).
On evaluation in the emergency center of the adjacent hospital, dyspnea and decreased mentality were observed (Glasgow Coma Scale score 10). After intubation, pinkish and frothy endotracheal secretions were identified, suggestive of pulmonary hemorrhage.
When the patient visited our hospital, his initial vital signs showed blood pressure 111/60 mm Hg, heart rate 119 beats per minute, respiratory rate 20 cycles per minute, and body temperature 37.0℃. However, several hours after arrival, his blood pressure decreased to 64/50 mm Hg, for which an infusion of norepinephrine was started. His initial oxygen saturation was 76% on monitoring. Both pupils responded normally to light and no abnormalities were noted in the conjunctivae and sclerae. Forty hours after the onset of dyspnea, a petechial rash was identified on the left upper chest wall and left axillary region. On auscultation, rales were heard on both upper lung fields and heart sounds were regular with no murmur. Abdominal palpation revealed no signs of hepatomegaly or splenomegaly. Several wounds from the liposuction surgery were clear without signs of infection.
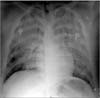
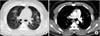
At the first visit to our hospital, the patient was intubated and oxygen was supplied by manual Ambu bagging. Initial arterial blood gas analysis showed pH 7.430, PaCO2 29.8 mm Hg, PaO2 57.7 mm Hg, HCO3- 19.4 mmol/L, and SaO2 91.1%. There was bilateral diffuse haziness on a chest radiograph (Figure 1), and a chest CT similarly showed bilateral diffuse airspace opacities and interlobular septal thickening (Figure 2). Integration of the patient history, radiologic findings, and symptoms of hemoptysis and pinkish secretion from the endotracheal tube was strongly suspicious of ARDS due to diffuse alveolar hemorrhage by FES. Initial laboratory parameters showed a total leukocyte count 6,400/mm3 (neutrophils, 89.7%; lymphocytes, 8.8%; monocyte, 1.4%), hemoglobin 17.0 g/dL, platelets 187,000/mm3, and C-reactive protein 0.45 mg/dL. All other blood chemistry parameters were within normal limits. The patient's coagulation profile was checked after transfer to our hospital. Prothrombin time was 14.5 seconds (international normalized ratio, 1.35) and the serum D-dimer level was 2.37 mg/L (normal range, <0.80 mg/L). Other investigations included urinalysis and microbiology testing. Fat globules were not detected in the urine and the final blood and sputum culture results revealed no isolated pathogenic organisms. The basic cardiac rhythm on electrocardiography was regular sinus tachycardia. On transthoracic echocardiography (TTE) to evaluate the cause of hypotension, right ventricular enlargement and signs of pulmonary hypertension were noted suggestive of right ventricular failure accompanied by FES. Dilation of the left ventricle with decreased contractile function (ejection fraction, 47%) was also noted.
Under the impression of fulminant FES, supportive care was provided that consisted of mechanical ventilation and prophylactic antibiotics. Several hours after hospital arrival, the patient's condition was aggravated by a systolic blood pressure decrease to 64 mm Hg and fever of 39.1℃. The operative wounds were clear and no other sources of infection leading to septic shock were found. Therefore, there seemed to be a low probability of septic shock. Acute heart failure in fulminant FES is thought to be a cause of hypotension. Norepinephrine was started and continuously infused to maintain blood pressure.
Three days after admission, the patient's medical condition and mentality had improved and the norepinephrine infusion was ceased. His respiratory function gradually recovered and he was successfully weaned from the ventilator on day 6. A repeat chest CT on day 10 revealed much clearing of the bilateral alveolar opacities (Figure 3). A subsequent echocardiographic evaluation performed on day 13 revealed a normalized left ventricular chamber size and recovered left ventricular contractile function to the lower normal limit. The right ventricular systolic pressure was decreased to 28 mmHg, indicating resolution of the pulmonary arterial hypertension. The patient showed no more symptoms except for a yellowish sputum and was discharged 14 days after admission.
Fat embolism is fat within the circulation, which can produce embolic phenomena with or without clinical sequelae4. FES is a relatively common complication after pelvic and long bone fracture and is commonly seen after procedures or conditions such as orthopedic surgery, severe burns, liver injury, closed-chest cardiac massage, and liposuction1. FES is fat in the circulation associated with an identifiable clinical pattern of symptoms and signs4. Its clinical manifestations include acute lung injury, altered mental status, petechiae or hemoptysis, central venous occlusion of the retina, retinal hemorrhage, and renal failure1. Non-traumatic conditions including acute pancreatitis, fatty liver, corticosteroid therapy, fat emulsion infusion, and hemoglobinopathies are very uncommon causes of FES5. Although the precise mechanisms of FES remain unclear, mechanical and biochemical theories have been proposed234.
In the mechanical theory, proposed in the 20th century, fat globules released from the bone marrow of a fracture site cause vessel occlusion that leads to organ failure6. However, it does not sufficiently explain the 24-72-hour interval following acute insult during which the patient is symptom free and does not explain non-traumatic FES.
The biochemical theory suggests that the production of toxic intermediaries (including free fatty acids and C-reactive protein) of plasma-derived fat may occur instead of or in addition to the mechanical fat embolism mechanism. Such toxic intermediaries directly injure alveolar and endothelial cells within lungs7.
The clinical findings are the cornerstone for diagnosing FES. Laboratory, radiological, and electrocardiographic findings in FES are either too sensitive or not specific enough to be pathognomonic for FES. The classic triad of FES involves pulmonary changes, cerebral dysfunction, and petechial rash. If this triad occurs 1-2 days after trauma, it is virtually pathognomonic for FES8. The most commonly used set of major and minor diagnostic criteria are those published by Gurd and Wilson9. The major criteria are based on the classic triad and clinical diagnosis is made by the presence of respiratory insufficiency, neurological impairment, and a petechial rash. For the diagnosis of FES, at least two major criteria or one major and four minor criteria must be present with fat macroglobulinemia.
A fat embolism index was recently proposed as a semiquantitative means of diagnosing FES, for which there are seven clinical features10. Each one is given a particular score. A score >5 is required for a positive diagnosis. Several radiological findings may be helpful for the diagnosis of FES. On a chest CT scan, bilateral ground-glass opacities, thickening of the interlobular septa, and centrilobular nodular opacities in lungs are present in some patients11.
In this case, the patient was diagnosed with FES on the basis of clinical and radiological findings. Three major criteria (hypoxemia, neurological impairment, petechiae) and two minor criteria were met in Gurd's diagnostic criteria, and the fat embolism index score was 15. Bilateral diffuse airspace opacity and pulmonary edema were observed on an initial chest CT scan without evidence of pulmonary thromboembolism.
Clinicians should distinguish between acute fulminant FES and other FES. Acute fulminant FES occurs during the first 24 hours and is attributed to massive mechanical blockage pulmonary vasculature by the fat emboli12. The clinical course is strongly dependent upon the quantity and rapidity of fat droplets occupying the pulmonary vessels. In fulminant FES, a sudden massive obstruction of the pulmonary circulation causes a rapidly increased impendence of the right ventricular ejection13, and pulmonary arterial pressure increases due to reactive vasoconstriction following hypoxemia14. By these mechanisms, pulmonary fat embolism is a life-threatening event that may potentially result in right ventricular failure.
In this case, the patient's cardiac function was measured and observed by TTE. At the initial echocardiographic evaluation, the measured right ventricular systolic pressure was 37 mm Hg, suggesting borderline pulmonary arterial hypertension, and right ventricular enlargement was also noted. These findings could be inferred from increased pulmonary vascular resistance caused by fat embolism. However, in addition to the right ventricular change, a moderately dilated left ventricular chamber and left ventricular contractile dysfunction were noted with diffuse global hypokinesia of the left ventricular wall (ejection fraction, 47%) suggestive of dilated cardiomyopathy. This is a notable difference from other cases of FES with right ventricular failure. Electrocardiographically, there were no abnormalities suggestive of ischemic injuries and the cardiac biomarkers were within normal limits. Coronary angiography to exclude ischemic insult, possibly resulting in left ventricular dysfunction, was not conducted due to the severity of the patient's condition.
Supportive care is the mainstay of therapy for clinically apparent FES15, and most patients with FES fully recover. Different approaches including corticosteroids, aspirin, and heparin have been tried with varying degrees of success. However, the small groups of patients studied to date make the results of many of these trials difficult to interpret. The most important factor in the treatment of FES is early resuscitation and stabilization to minimize the stress response and hypovolemia4. The evidence of routine use of corticosteroids in patients with FES is not sufficient15. Considering the concern of severe infection, we did not use systemic corticosteroids for the treatment of fat embolism. Exceptionally, hydrocortisone 100 mg was administered daily for 3 days as a pre-medication for immunoglobulins.
To the best of our knowledge, this is the first report of the occurrence of fulminant fat embolism as a complication of liposuction surgery in Korea. With its clinically rapid progression, the patient demonstrated symptoms of multiple organ failure (involving the respiratory, cardiovascular, and central nervous systems) but fully recovered. As the demand for cosmetic surgery continues to increase, especially in younger adults, the number of aesthetic surgeries performed in Korea increases annually. Although the exact frequency of FES after liposuction surgery has not been reported until now, it is expected to increase with the procedure demand. Therefore, clinicians should consider the possibility of FES in the post-operative period of liposuction surgery.
Figures and Tables
Acknowledgements
The authors thank Tae Hyun Ban, M.D., for collecting data and providing study advice.
References
1. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007; 37:5–8.
2. Karagiorga G, Nakos G, Galiatsou E, Lekka ME. Biochemical parameters of bronchoalveolar lavage fluid in fat embolism. Intensive Care Med. 2006; 32:116–123.
3. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop (Belle Mead NJ). 2002; 31:507–512.
4. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001; 56:145–154.
5. Shapiro MP, Hayes JA. Fat embolism in sickle cell disease: report of a case with brief review of the literature. Arch Intern Med. 1984; 144:181–182.
6. Warthin AS. Traumatic lipaemia and fatty embolism. Ann Arbor: University of Michigan;1913.
7. Akhtar S. Fat embolism. Anesthesiol Clin. 2009; 27:533–550.
8. Baselga J, Reich L, Doherty M, Gulati S. Fat embolism syndrome following bone marrow harvesting. Bone Marrow Transplant. 1991; 7:485–486.
9. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974; 56:408–416.
10. Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroids: a prospective study in high-risk patients. Ann Intern Med. 1983; 99:438–443.
11. Malagari K, Economopoulos N, Stoupis C, Daniil Z, Papiris S, Muller NL, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003; 123:1196–1201.
12. Bajuri MY, Johan RR, Shukur H. Two variants of fat embolism syndrome evolving in a young patient with multiple fractures. BMJ Case Rep. 2013; 2013:bcr2013008631.
13. Pitto RP, Blunk J, Kossler M. Transesophageal echocardiography and clinical features of fat embolism during cemented total hip arthroplasty: a randomized study in patients with a femoral neck fracture. Arch Orthop Trauma Surg. 2000; 120:53–58.
14. Ward JP, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respir Physiol. 1999; 115:261–271.
15. Ashbaugh DG, Petty TL. The use of corticosteroids in the treatment of respiratory failure associated with massive fat embolism. Surg Gynecol Obstet. 1966; 123:493–500.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download