Abstract
The prevalence of lung diseases caused by nontuberculous mycobacteria (NTM) is increasing worldwide. Unlike pulmonary tuberculosis, endobronchial NTM diseases are very rare with the majority of cases reported in patients with human immunodeficiency virus infection and acquired immune deficiency syndrome. We reported a rare case of endobronchial Mycobacterium avium disease associated with lobar atelectasis in a young immunocompetent patient and reviewed the relevant iterature.
Nontuberculous mycobacteria (NTM) generally refer to mycobacteria other than Mycobacterium tuberculosis complex and Mycobacterium leprae. The prevalence of lung diseases caused by NTM is increasing worldwide, including in South Korea12. NTM lung disease commonly occurs in patients with structural lung disease, such as with prior tuberculosis and bronchiectasis3.
Endobronchial tuberculosis is a well-described manifestation of pulmonary tuberculosis in immunocompetent patients and is associated with significant local complications4. However, endobronchial NTM diseases are rare and the majority of cases have been reported in patients with human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS)5678910. Endobronchial NTM diseases are rare in non-HIV/AIDS patients. To the best of our knowledge, there have only been four case reports of isolated endobronchial NTM diseases in immunocompetent adult patients in the English literature11121314. Here, we report a unique case of endobronchial Mycobacterium avium disease associated with lobar atelectasis in a young immunocompetent patient and provide a review of the literature.
A 37-year-old woman was referred to our hospital because she had exhibited dry cough for 1 month. She had been a healthy non-smoker up to this point, with the exception of having pulmonary tuberculosis 17 years prior. She had no history of alcoholism or use of immunosuppressive drugs.
Physical examination showed that the patient was 155.6 cm tall and weighed 50.8 kg. The results of the clinical laboratory tests were unremarkable with the exception of an elevated white blood cell count (10,460/µL), platelet level (546,000/µL), and erythrocyte sedimentation rate (61 mm/hr). The level of serum C-reactive protein was normal (0.09 mg/dL). A HIV antibody test was negative.
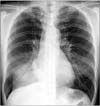
Chest radiography revealed a dense consolidation in the right lower zone (Figure 1). Computed tomography scan of the chest showed volume loss of the right lower lobe associated with consolidation and mucus retention of lung parenchyma (Figure 2A) and a hypertrophied subcarinal lymph node (Figure 2B). Bronchoscopy revealed an ulcerative lesion at the medial side of the right main bronchus (Figure 3) and a bronchial stricture of the right bronchus intermedius and the right lower lobe. Bronchial washing fluid was negative for acidfast bacilli and nucleic acid amplification test for M. tuberculosis was also negative. Bronchial biopsy at the right main bronchus revealed chronic granulomatous inflammation with necrosis. Nucleic acid amplification test for M. tuberculosis in the formalin-fixed, paraffin-embedded biopsy specimens was negative. However, NTM was isolated in both liquid and solid media from the bronchial washing specimens and M. avium was identified.
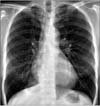
The patient was diagnosed with endobronchial M. avium disease and received antibiotic therapy including azithromycin 250 mg/day, rifampin 600 mg/day, and ethambutol 800 mg/day for 15 months3. The patient's symptoms disappeared and the atelectasis of the right lower lobe improved on chest radiography after 12 months of antibiotic treatment (Figure 4).
The prevalence of lung disease due to NTM is increasing worldwide, affecting both immunocompetent and immunocompromised individuals12. M. avium complex, Mycobacterium abscessus complex, and Mycobacterium kansasii are the most frequent pathogens involved in NTM lung disease3. The traditionally recognized clinical presentation of NTM lung disease is apical fibrocavitary disease. NTM lung disease can also present with nodular infiltrates, frequently involving the right middle lobe and the lingular segment of the left upper lobe. This form of disease is termed nodular bronchiectatic disease15.
Although the pulmonary parenchymal presentation of NTM has been well-described, there have been only four case reports of isolated endobronchial NTM infections in immunocompetent patient11121314. Our case report is the fifth cases of isolated endobronchial NTM infection in an immunocompetent patient. The details of the five cases, including the present case, are presented in Table 1. We found that four out of the five patients were in their 50s and three of the five were female. M. avium was isolated in three cases and M. kansasii in two cases. Antibiotic treatment for each case of NTM was initiated and four out of the five patients showed improvement in their endobronchial lesion11121314.
Endobronchial NTM infection and endobronchial tuberculosis requires rapid diagnosis and treatment because they can progress to bronchial stenosis. Bronchoscopy is useful for diagnosing endobronchial tuberculosis and typically reveals edematous mucosa, granular features, inflammatory polyps, and ulcers4. The bronchoscopic findings of endobronchial NTM disease are similar to those of endobronchial tuberculosis11121314. The proper management of isolated endobronchial NTM disease remains unclear. In our case, good treatment outcome was achieved with the recommended combination antibiotic therapy without bronchoscopic intervention.
In conclusion, we report a unique case of endobronchial NTM disease caused by M. avium in a young immunocompetent adult patient. Bronchoscopy is useful for the diagnosis of endobronchial NTM disease. Combination antibiotic therapy provided symptomatic relief and improvement of the endobronchial lesions and associated lobar atelectasis.
Figures and Tables
 | Figure 2(A) An axial chest computed tomography (CT) image at the level of the left atrium shows lobar consolidation of the right lower lobe. Note the fluid bronchograms in the dilated bronchi . (B) An axial chest CT image at the level of the right bronchus intermedius shows a hypertrophied and well-enhancing subcarinal lymph node (arrow). Note this lymph node attaches to the right main bronchus. |
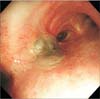 | Figure 3A bronchoscopic image demonstrating an ulcerative endobronchial lesion on the medial side of the right main bronchus. |
Table 1
Isolated endobronchial nontuberculous mycobacterial infection in non-HIV/AIDS adult patients in the English literature

| Author | Age (yr)/Sex | Organism | Associated findings | Treatment |
|---|---|---|---|---|
| Connolly et al. (1993)11 | 52/F | Mycobacterium kansasii | RUL cavity | INH RFP EMB, noncompliant |
| Fukuoka et al. (2003)12 | 57/M | M. avium | LUL mass | INH RFP EMB SPX CLR for 12 months, improvement |
| Manali et al. (2005)13 | 58/M | M. kansasii | Large calcified subcarinal lymph nodes, diffuse pleural thickening of the right hemithorax | INH RFP EMB for 18 months, improvement |
| Kang et al. (2013)14 | 59/F | M. avium | Multiple pulmonary infiltrates | CLR RFP EMB, improvement |
| Present study | 37/F | M. avium | Subcarinal lymphadenopathy and RLL atelectasis | AZM RFP EMB for 15 months, improvement |
Acknowledgements
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A120647) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2060552).
References
1. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:87–94.
2. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis. 2013; 75:199–204.
3. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
4. Jung SS, Park HS, Kim JO, Kim SY. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology. 2015; 20:488–495.
5. Packer SJ, Cesario T, Williams JH Jr. Mycobacterium avium complex infection presenting as endobronchial lesions in immunosuppressed patients. Ann Intern Med. 1988; 109:389–393.
6. Mehle ME, Adamo JP, Mehta AC, Wiedemann HP, Keys T, Longworth DL. Endobronchial Mycobacterium avium-intracellulare infection in a patient with AIDS. Chest. 1989; 96:199–201.
7. Cordasco EM Jr, Keys T, Mehta AC, Mehle ME, Longworth DL. Spontaneous resolution of endobronchial Mycobacterium avium-intracellulare infection in a patient with AIDS. Chest. 1990; 98:1540–1542.
8. Quieffin J, Poubeau P, Laaban JP, Brechot JM, Capron F, Rochemaure J. Mycobacterium kansasii infection presenting as an endobronchial tumor in a patient with the acquired immune deficiency syndrome. Tuber Lung Dis. 1994; 75:313–315.
9. Bartley PB, Allworth AM, Eisen DP. Mycobacterium avium complex causing endobronchial disease in AIDS patients after partial immune restoration. Int J Tuberc Lung Dis. 1999; 3:1132–1136.
10. Kim HC, Bae IG, Ma JE, Lee JS, Jeon KN, Lee JD, et al. Mycobacterium avium complex infection presenting as an endobronchial mass in a patient with acquired immune deficiency syndrome. Korean J Intern Med. 2007; 22:215–219.
11. Connolly MG Jr, Baughman RP, Dohn MN. Mycobacterium kansasii presenting as an endobronchial lesion. Am Rev Respir Dis. 1993; 148:1405–1407.
12. Fukuoka K, Nakano Y, Nakajima A, Hontsu S, Kimura H. Endobronchial lesions involved in Mycobacterium avium infection. Respir Med. 2003; 97:1261–1264.
13. Manali ED, Tomford WJ, Liao DW, Farver C, Mehta AC. Mycobacterium kansasii endobronchial ulcer in a nonimmunocompromised patient. Respiration. 2005; 72:305–308.
14. Kang SH, Mun SK, Lee MJ, Kim SY, Choi HG, Byun J, et al. Endobronchial Mycobacterium avium infection in an immunocompetent patient. Infect Chemother. 2013; 45:99–104.
15. Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis. 2014; 18:1141–1148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download