Abstract
Cryptogenic organizing pneumonia (COP) is an idiopathic interstitial pneumonia characterized by a subacute course and favorable prognosis with corticosteroids. However, some patients show resistance to steroids. Macrolides have been used with success in those patients showing resistance to steroids. A few reports showed treatment failure with macrolides in patients with COP who were resistant to steroids. In this report, we described two cases of COP who showed different responses to clarithromycin. One recovered completely, but the other gradually showed lung fibrosis with clarithromycin.
Cryptogenic organizing pneumonia (COP) is characterized by subacute illness of cough, flulike illness, and dyspnea and classified as one of acute or subacute idiopathic interstitial pneumonias (IIP). COP is discriminated with secondary organizing pneumonia which appears with collagen vascular disease, infection, and drug reaction. Diagnosis of COP can be made in the absence of associated such diseases. The histopathologic pattern is characterized primarily by organizing pneumonia involving alveolar ducts and alveoli with or without bronchial intraluminal polyps. High resolution computerized tomogram (HRCT) findings are nonspecific and usually includes patchy and often migratory consolidation in a subpleural, peribronchial, or band-like pattern1.
Majority of patients recover completely with oral steroids, but some patient dose not respond to long-term use of steroid. Furthermore, relapses after discontinuing of steroid, residual and progressive interstitial fibrosis were observed in small number of patients2. For patients who do not respond to steroid, macrolides (clarithromycin or erythromycin) and cyclosporine A has been used for alternate treatment345. Few reports described treatment failure with clarithromycin in patients with COP who did not respond to steroid.
Herein, we report two cases of COP who showed difference responses to clarithromycin. One recovered completely, but the other gradually showed lung fibrosis with clarithromycin.
A 63-year old woman appeared with a 2-month history of aggravating dyspnea on exertion (modified British Medical Research Council, mMRC II). She denied any exposure to chemical agents, know toxin, and dusts. She had no symptoms related with rheumatic and collagen vascular diseases like arthralgia, skin rash, dry mouth, and Raynaud's phenomenon. She was never-smoker and a teacher of kindergarten. Physical examination showed no cyanosis and no fever, tachypnea (24/min), and bilateral inspiratory crackles on both lung fields.
Chest radiography showed irregular and patchy air-space consolidations in both lungs, more predominantly seen in basal and peripheral lungs (Figure 1) and HRCT showed peribronchial and subpleural patchy air space consolidation with ground glass attenuation (GGA), mostly located peripheral and lower lung areas (Figure 2). The hemoglobin was 13.1 g/dL, white blood cell count was 8,400/µL (neutrophil, 59.4%; lymphocyte, 27.3%; monocyte, 10.1%; eosinophil, 2.4%; basophil, 0.8%), platelet 264,000/µL, erythrocyte sedimentation rate (ESR) 35 mm/hr, and C-reactive protein 0.40 mg/dL. Arterial blood gas analysis showed pH 7.432, PaCO2 29.9 mm Hg, PaO2 75.7 mm Hg, SaO2 95.7%, and HCO3- 19.5 mmol/L. Pulmonary function test showed a forced vital capacity (FVC) of 1.86L/62% of the predicted value, forced expiratory volume in 1 second (FEV1) 1.44L/66% of the predicted value, FEV1/FVC ratio of 77%, diffusing lung capacity of carbon monoxide (DLCO) of 53% of predicted value, and DLCO/volume of alveolar 80% of predicted value. Cellular analysis of bronchoalveolar lavage (BAL) fluid revealed 55% of macrophages, 15% of neutrophils, and 25% of lymphocytes. No microbiological agents or malignancy were found. Antinuclear antibodies (ANA), anti-double stranded DNA, complement C3, C4, and angiotensin converting enzyme (ACE) were negative or within normal limits. And IgG, IgM, and IgA were all negative. Serology blood tests and other autoimmunity studies did not point any collagen vascular and autoimmune diseases. Echocardiogram showed no abnormal findings with ejection fraction (EF) of 76%.
Lung biopsy through video-assisted thoracoscopic surgery (VATS) was performed to differentiate COP, nonspecific interstitial pneumonia (NSIP), and resolving bacterial pneumonia on the third hospital day. Histology of lung biopsy showed anastomosing polypoid plugs of loose connective tissue protruding into the alveolar ducts and spaces. The alveolar walls are mildly thickened and interstitial inflammation is moderate (Figure 3). In addition, the lung tissue obtained by lung biopsy was submitted for cultures and specialized stain to detect organism, but none of tissue cultures and stains were positive.
Therapy was started with prednisolone (PDL, total 50 mg, 1 mg/kg/day) on 13th hospital day. Her symptoms, FVC, and HRCT were worsened after 1-month treatment of PDL. We added cyclophosphamide (total 100 mg, 2 mg/kg/day) for next 1 month together with PDL, but HRCT (Figure 4A), her symptoms, FVC worsened. Clarithromycin 500 mg was added with reduced dose of PDL. Cyclophosphamide was stopped with start with clarithromycin. At three months after treatment with clarithromycin, HRCT (Figure 4B), her symptoms (mMRC I), and FVC (63% of predicted value) much improved. After 6 months of stopping PDL and clarithromycin, she complained no respiratory symptoms, HRCT and FVC (81% of predicted value) became to really normal (Figure 4C).
An 81-year-old woman complained of exertional dyspnea (mMRC II) and general weakness for 2 weeks. She denied any exposure to chemical agents, known toxin, and dusts. She had no symptoms related with rheumatic and collagen vascular diseases like arthralgia, skin rash, dry mouth, and Raynaud's phenomenon. She was never-smoker and a house-wife. Physical examination showed no cyanosis, afebrile, tachypnea (26/min), and bilateral inspiratory crackles on both lung fields.
Chest radiography showed peribronchiolar consolidation in bilateral lower lobes and lingular division. Air-bronchogram was observed in lingular division (Figure 5). HRCT showed multifocal areas of subpleural or peribronchial consolidations in both lungs with combined small amount of pleural effusion (Figure 6). The hemoglobin was 13.1 g/dL, white blood cell count 10,800/µL (neutrophil, 73.5%; lymphocyte, 17.3%; monocyte, 6.0%; eosinophil, 2.4%; basophil, 0.8%), platelet 181,000/µL, ESR 45 mm/hr, and C-reactive protein 0.67 mg/dL. Arterial blood gas analysis showed pH 7.454, PaCO2 28.7 mm Hg, PaO2 48.6 mm Hg, SaO2 86.9%, and HCO3- 19.7 mmol/L. Pulmonary function test showed a FVC of 1.19 L/63% of the predicted value, FEV1 1.03 L/85% of the predicted value, and FEV1/FVC ratio of 87%. Cellular analysis of BAL fluid revealed 50% of macrophages, 17% of neutrophils, and 26% of lymphocytes. No microbiological agents or malignancy were found. ANA, anti-double stranded DNA, complement C3, C4, and ACE were negative or within normal limits. And IgG, IgM, and IgA were all negative. Serology blood tests and other autoimmunity studies did not point any collagen vascular and autoimmune diseases.
The differential diagnosis included IIP such as NSIP and COP, bacterial pneumonia, and heart failure. Echocardiogram showed no abnormal findings with EF of 79%. We decided to perform surgical lung biopsy to know exact diagnosis in spite of relatively old age. VATS was performed on the seventh hospital day. Histology of lung biopsy showed polypoid plugs of loose organizing connective tissue within respiratory bronchioles, alveolar ducts and spaces. The alveolar walls are mildly thickened and interstitial inflammation is relatively mild. These findings suggested COP (Figure 7). In addition, the lung tissue obtained by lung biopsy was submitted for cultures and specialized stain to detect organism, but none of tissue cultures and stains were positive.
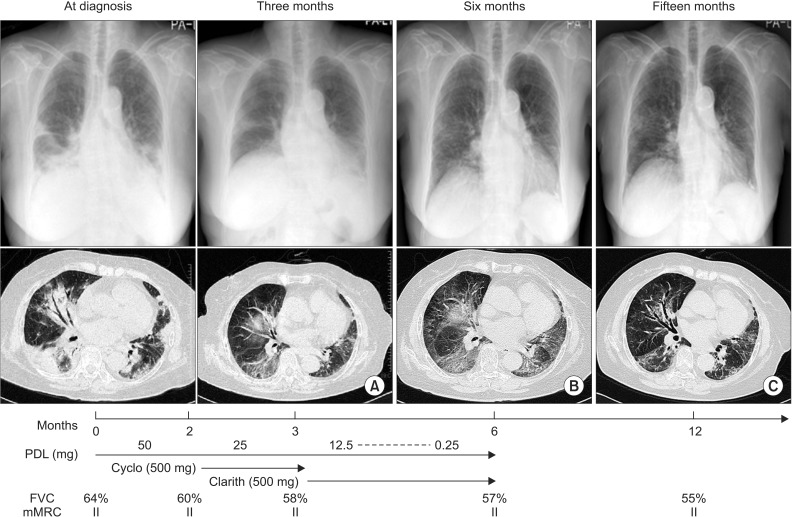
Therapy was started with PDL (total 50 mg, 1 mg/kg/day) on 14th hospital day. Her exertional dyspnea was not changed, but FVC was decreased to 60% after 2 months treatment of PDL. We added cyclophosphamide (total 100 mg, 2 mg/kg/day) for next 1 month together with PDL, but her symptoms (mMRC II), FVC (58% of predicted value) were not improved. HRCT showed still consolidation and GGA (Figure 8A). Clarithromycin 500 mg was added with reduced dose of PDL and cyclophosphamide was stopped. At 3 months after treatment with clarithromycin, her symptoms (mMRC II) and FVC (57% of predicted value) still were not improved and HRCT showed no change of consolidation and GGA (Figure 8B). At 6 months after discontinuing of medication, her symptoms (mMRC II) and FVC (55% of predicted value) were not improved. Her HRCT showed still GGA and fibrotic changes (Figure 8C).
In the present study, we showed clarithromycin-responsive and -resistant patients with COP who refractory to steroid. Generally, steroid in COP showed rapid resolution within several days or a few weeks and dramatic effect of majority patients. But, both of patients were not response to steroid 2 or 3 months, thus we used alternative therapy, macrolides.
The failure of treatment with clarithromycin was rarely reported. Almost all reports described good response to clarithromycin in patients with COP4567. Therefore we believe clarithromycin is good alternative drugs without failure in treatment of COP. Radzikowska et al.8 reported that the response rate of clarithromycin was 75% in 12 patients with organizing pneumonia; however, the population of patients in this study was mixed with COP and secondary organizing pneumonia (OP). One patient failed to respond to intravenous erythromycin, but the duration of treatment was only 3 days9. Therefore, we don't know yet the failure rate of macrolides in the treatment of COP.
The mechanism of macrolides in the treatment of COP is anti-inflammatory effects rather than antibiotic effects. Although the underlying mechanisms of anti-inflammatory effects are unknown, in vitro and in vivo studies suggest anti-inflammatory effects of macrolides may be related with the effect on polymorphonuclear cells and their products but also effect on T cells. Erythromycin decrease the number of neutrophils and the neutrophil-derived elastolytic-like activity in BAL fluid decreased significantly after treatment with erythromycin in patients with bronchiolitis10. Macrolides treatment for 1-24 months significantly reduced BAL fluid levels of interleukin (IL) 1β and IL-8 in parallel with BAL fluid neutrophils in patients with diffuse panbronchiolitis11. Furthermore, erythromycin decreased significantly the number of neutrophils recovered from lungs of mice responding to bacterial challenge12. Erythromycin was capable of inhibiting expression of the IL-8 genes in T cells through transcriptional inhibition13. In the presents cases, the level of neutrophils and lymphocyte in BAL fluid were not different between clarithromycin- responsive (case 1) and -nonresponsive (case 2) cases. These findings suggested the level of neutrophils and lymphocytes do not affect the response to clarithromycin, although only two cases were analyzed.
The discrepancy between cellular profiles in BAL fluid and histologic finding of lung biopsy was found in present cases. The level of neutrophils in BAL fluid was 13% and 15% in case 1 and 2, respectively, but, neutrophils in histology of lung biopsy were not observed as the level of BAL finding. This discrepancy may be explained by proximal bronchial airway obstruction preventing normal saline reaching diseased lung during BAL procedure.
Cohen et al.14 reported OP with rapidly progressive clinical course and poor outcome. The majority of these patients had secondary OP associated with autoimmune disorders. This finding suggested the presence of autoimmune and connective tissue disorders were important predictor of rapidly progressive OP. However, also, in case of COP, rapidly progressive and fatal patients were reported9. In our cases, both patients did not show any evidences of associated diseases.
The duration of macrolides in patients with COP was unknown yet. However, the majority of cases were successfully treated more than 3 months of macrolides without relapse347. One case of patient showed relapse of COP after a 3-week course was discontinued7, and the other case showed no improvement of clinical condition9. These finding suggested long-term use more than three months of macrolides are needed for treatment and prevention of relapse. In our cases, case 1 showed significant clinical improvement after 3-month course of medication and no relapse after discontinuation, but case 2 showed no clinical responses in spite of 3 months of clarithromycin. Further studies are needed to decide when we should stop macrolides in case of no-responsive to these drugs.
Laboratory abnormalities, such as severe anemia (Hb less than 11 g/dL), high ESR (more than 60 mm/hr), and low serum albumin (less than 3.5 g/dL) could be associated with worse prognosis in 61 patients with COP and secondary OP1. And paucity of lymphocytes in BAL fluid was suggested as a risk factor for relapse. In present case, case 2 showed no abnormalities in anemia, ESR, and albumin level, and also lymphocytes in BAL fluid were similar level as case 1. Therefore, case 2 showed no risk factor for worsening of COP.
Generally, the disease severity of idiopathic pulmonary fibrosis is assessed on the basis of symptoms, pulmonary function test, and radiologic findings15. We applied these parameters to response to treatment in both two cases. We regarded improvement of disease in case of at least two of the above three parameters improved. In case 1, after use of clarithromycin showed improvement of every parameter, FVC, mMRC, and HRCT (Figure 4). But, in case 2, any drugs could not improve these parameters (Figure 8).
There is remarkable point that the age of the two patients was quite different. So, we suggest that age of patient may affect the results because of both patient's underlying immunological condition, lung biopsy setting, HRCT, initial FVC, and mMRC were similar.
In conclusion, we herein report clarithromycin-responsive and -resistant patients with COP who refractory to steroid. This clarithromycin-resistant case indicates that there is a need to evaluate the failure rate of macrolides including clarithromycin in treatment of COP.
References
1. Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, Aswad B, Karagianidis N, Kastanakis E, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest. 2011; 139:893–900. PMID: 20724743.
2. Yousem SA, Lohr RH, Colby TV. Idiopathic bronchiolitis obliterans organizing pneumonia/cryptogenic organizing pneumonia with unfavorable outcome: pathologic predictors. Mod Pathol. 1997; 10:864–871. PMID: 9310948.
3. Chang WJ, Lee EJ, Lee SY, In KH, Kim CH, Kim HK, et al. Successful salvage treatment of steroid-refractory bronchiolar COP with low-dose macrolides. Pathol Int. 2012; 62:144–148. PMID: 22243785.

4. Ichikawa Y, Ninomiya H, Katsuki M, Hotta M, Tanaka M, Oizumi K. Low-dose/long-term erythromycin for treatment of bronchiolitis obliterans organizing pneumonia (BOOP). Kurume Med J. 1993; 40:65–67. PMID: 8231065.

5. Lee J, Cha SI, Park TI, Park JY, Jung TH, Kim CH. Adjunctive effects of cyclosporine and macrolide in rapidly progressive cryptogenic organizing pneumonia with no prompt response to steroid. Intern Med. 2011; 50:475–479. PMID: 21372463.

6. Adibelli Z, Dilek M, Kocak B, Tulek N, Uzun O, Akpolat T. An unusual presentation of sirolimus associated cough in a renal transplant recipient. Transplant Proc. 2007; 39:3463–3464. PMID: 18089408.

7. Stover DE, Mangino D. Macrolides: a treatment alternative for bronchiolitis obliterans organizing pneumonia? Chest. 2005; 128:3611–3617. PMID: 16304320.
8. Radzikowska E, Wiatr E, Gawryluk D, Langfort R, Bestry I, Chabowski M, et al. Organizing pneumonia: clarithromycin treatment. Pneumonol Alergol Pol. 2008; 76:334–339. PMID: 19003763.
9. Nizami IY, Kissner DG, Visscher DW, Dubaybo BA. Idiopathic bronchiolitis obliterans with organizing pneumonia: an acute and life-threatening syndrome. Chest. 1995; 108:271–277. PMID: 7606970.
10. Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, et al. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1992; 146:196–203. PMID: 1626803.

11. Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996; 63:42–48. PMID: 8833992.
12. Nelson S, Summer WR, Terry PB, Warr GA, Jakab GJ. Erythromycin-induced suppression of pulmonary antibacterial defenses: a potential mechanism of superinfection in the lung. Am Rev Respir Dis. 1987; 136:1207–1212. PMID: 3314615.

13. Aoki Y, Kao PN. Erythromycin inhibits transcriptional activation of NF-kappaB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrob Agents Chemother. 1999; 43:2678–2684. PMID: 10543746.
14. Cohen AJ, King TE Jr, Downey GP. Rapidly progressive bronchiolitis obliterans with organizing pneumonia. Am J Respir Crit Care Med. 1994; 149:1670–1675. PMID: 8004328.

15. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011; 183:788–824. PMID: 21471066.
Figure 1
Chest posterior-anterior (A) and left lateral (B) X-ray showed irregular and patchy air-space consolidations in both lungs, more predominantly seen in the basal and peripheral lungs.
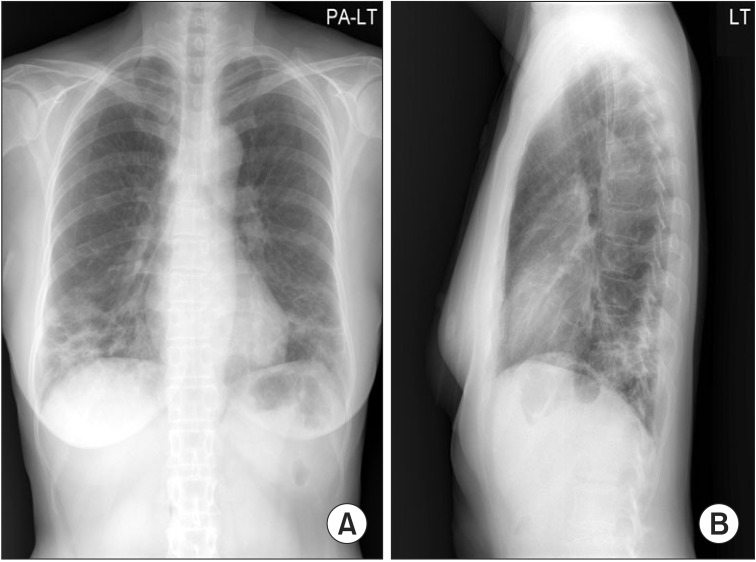
Figure 2
(A-D) High resolution computerized chest tomogram showed peri bronchial and subpleural patchy air space consolidation with ground-glass att enuation, mostly located at the peripheral and lower lung areas.
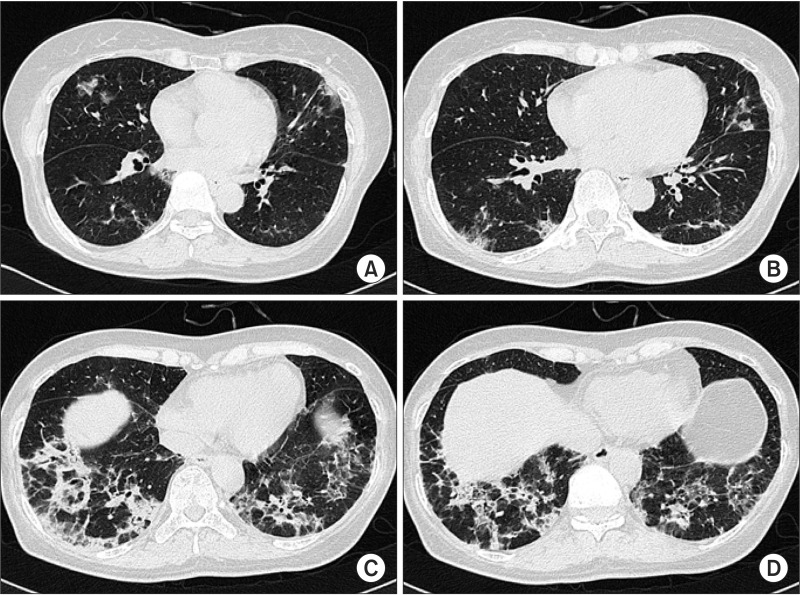
Figure 3
Microscopic examination shows anastomosing polypoid plugs of loose connective tissue protruding into the alveolar ducts and spaces. The alveolar walls are mildly thickened and interstitial inflammation is moderate (H&E stain, ×100).
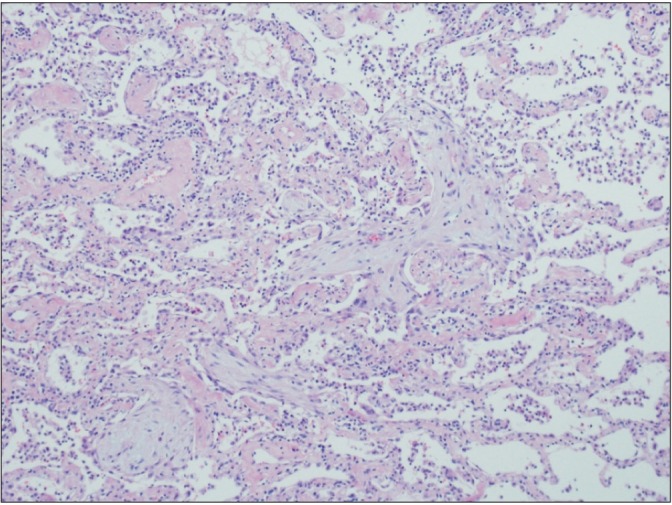
Figure 4
Summary of treatment agents, clinical course, and radiologic findings. High resolution chest computerized tomogram of a case that worsened after 2 months treatment of prednisolone and 1-month treatment of cyclophosphamide (A), and improved with 3 months treatment with clarithromycin (B). High resolution computerized tomogram, lung function tests, and dyspnea improved 6 months after ceasing administration of clarithromycin (C). PDL: prednisolone; Cyclo: cyclophosphamide; Clarith: clarithromycin; FVC: forced vital capacity; DLco: diffusion capacity of lung; mMRC: modified British Medical Research Council.
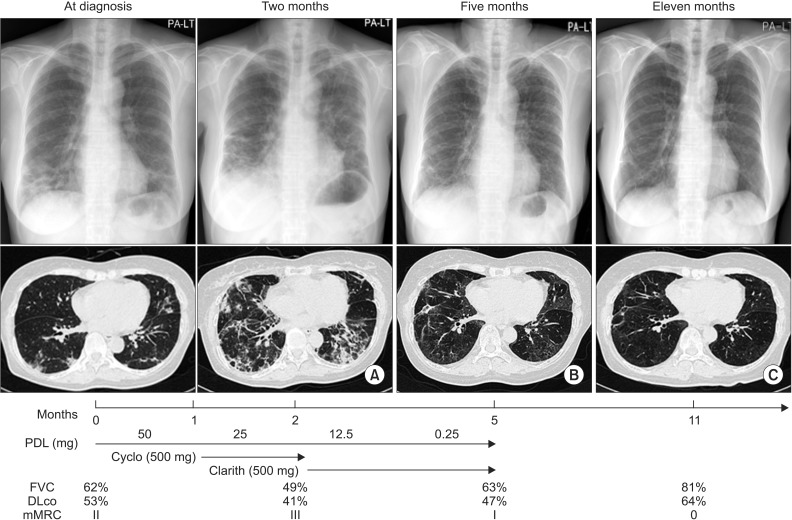
Figure 5
Chest posterior-anterior showed peribronchiolar consolidation in the bilateral lower lobes and lingular division. Airbronchogram was observed in the lingular division.
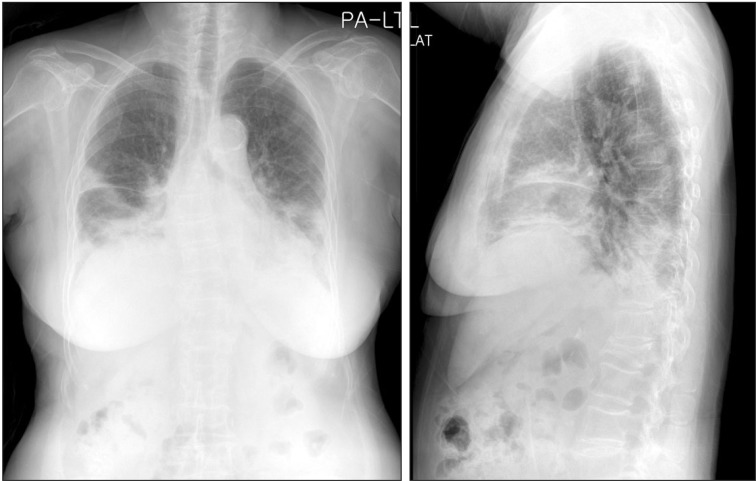
Figure 6
(A-D) High resolution computerized chest tomogram showed multifocal areas of subpleural or peribronchial consolidations in both lungs with a combined small amount of pleural effusion.
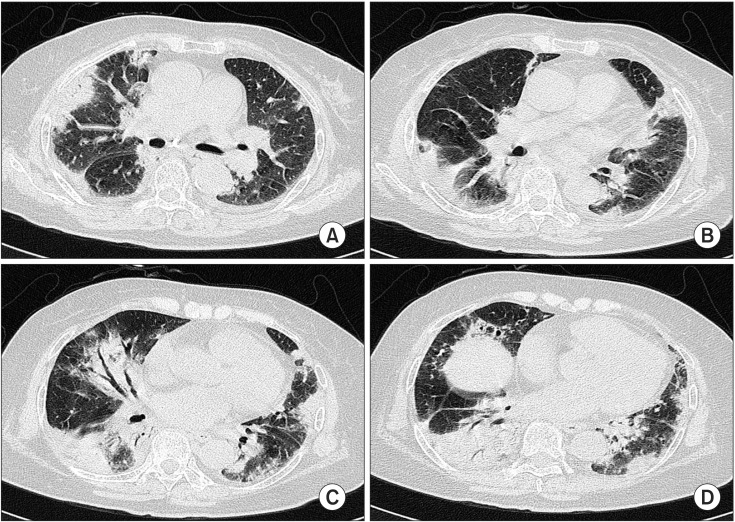
Figure 7
Microscopic examination shows polypoid plugs of loose organizing connective tissue within respiratory bronchioles, alveolar ducts and spaces. The alveolar walls are mildly thickened and the interstitial inflammation is relatively mild.
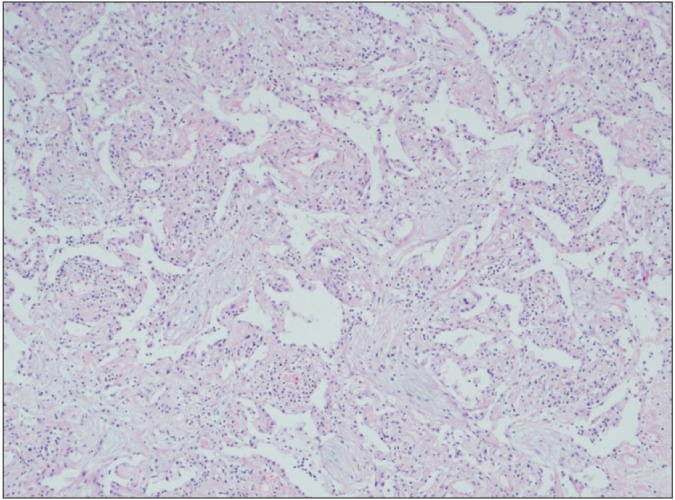
Figure 8
Summary of treatment agents, clinical course, and radiologic findings. High resolution chest computerized tomogram (HRCT) of a case that was improved in consolidations, but still with consolidation and ground glass attenuation (GGA) were observed after 3 months treatment of predniolone and 1-month treatment of clarithromycin (A). After three months treatment with clarithromycin, HRCT showed no change in consolidation and GGA (B). Six months after ceasing administration of clarithromycin, HRCT showed fibrotic change in both lung fields (C). PDL: prednisolone; Cyclo: cyclophosphamide; Clarith: clarithromycin; FVC: forced vital capacity; mMRC: modified British Medical Research Council.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download