Abstract
Systemic arterial supply from the descending thoracic aorta to the basal segment of the left lower lobe without a pulmonary arterial supply is a rare congenital anomaly within the spectrum of sequestration lung disease. The most common pattern of anomalous systemic artery to the lung arises from the descending thoracic aorta and feeds the basal segments of the left lower lobe. We report an extremely rare case of a 29-year-old woman who underwent a successful left upper lobectomy for the treatment of recurrent massive hemoptysis from anomalous bronchial arterial supply to the lingular segment of left upper lobe.
Anomalous systemic arterial supply to normal segments of the lung is a rare congenital anomaly. Cauldwell and Siekert1 performed an extensive anatomical study of the bronchial arteries in 150 human cadavers, and aberrant bronchial arteries were defined as arteries originating outside the descending thoracic aorta between the T5 and T6 vertebrae of the descending aorta, with an intrapulmonary course along the major bronchi. The reported prevalence of aberrant bronchial arteries ranges from 8.3% to 36.0%.
While many patterns of anomalous artery have also been sporadically reported23, an anomalous artery from descending aorta that supplied part of the left upper lobe, as in the current case, has not been reported previously.
Herein, we report the case of a young woman who underwent left upper lobectomy to control massive hemoptysis from anomalous bronchial artery to left lingular segment.
A 29-year-old woman came to the emergency room with massive hemoptysis, producing more than 300 mL of fresh blood. Approximately 6 weeks prior, she had developed sudden massive hemoptysis for the first time. Before the initial event, she did not have any previous medical history or medication history. She had gone to a neighboring hospital, and then undergone endoscopic hemostasis.
For further evaluation and management, she was referred to our hospital. On physical examination, the patient was afebrile, blood pressure was 100/70 mm Hg, heart rate was 80 beats per minute, respiratory rate was 20 breaths per minute, and oxygen saturation 98% on room air. Head and neck examination showed normal dentition with no lymphadenopathy. Chest examination showed clear breath sounds bilaterally with decreased air entry in the left lower lobe. Abdominal and cardiac exams were unremarkable. The results of a complete blood count were normal, except for hemoglobin (11.0 g/dL). An arterial blood gas analysis showed pH 7.41, pCO2 34 mm Hg, and PO2 79 mm Hg. Chest radiograph had no abnormal findings.
She experienced persistent dry cough with expectoration of more than 200 mL blood at once, and her hemoglobin level decreased from 11.0 g/dL to 9.5 g/dL. For management of massive hemoptysis, thoracic aortogram and left bronchial angiogram was performed, and it revealed aberrant bronchial artery with tortuous dilatation arising from the T9 vertebrae level of descending thoracic aorta and parenchymal blush of left upper lobe (Figure 1). Left aberrant bronchial artery was selected, and spinal artery branch was seen on bronchial angiography. Selective catheterization was performed for stabilization of the microcatheter within the bronchial artery and safe positioning in the bronchial circulation beyond the origin of spinal cord branches, an approach that prevents severe complication. Left aberrant bronchial artery embolization was performed successfully using a 3 mm×2 cm stainless steel platinum coil (Tornado; Cook Medical Inc., Bloomington, IN, USA). This procedure resulted in control of the hemoptysis, and no procedure-related complications occurred. She was discharged.
On her second visit, her hemoglobin level was 11.7 g/dL; however, her follow-up hemoglobin level dropped to 9.6 g/dL and revealed a blood pressure of 92/46 mm Hg and a pulse rate of 73 per minute. Chest radiograph showed focal increased parenchymal opacities on left lower lung field, and multidetector computed tomographic angiography (MDCTA) showed a dilated left bronchial artery arising from thoracic aorta, a finding that was suggestive of the bleeding focus due to blood clots in left upper lobar lingular segment, left lower lobar, and segmental bronchus (Figures 2, 3). Left aberrant bronchial artery arteriography still showed tortuous dilatation and marked parenchymal contrast stain. It was difficult to achieve a selective and stable catheterization within the bronchial artery. Moreover, angiographic findings showed subintimal dissection of the origin site of the left aberrant bronchial artery and extravasations of contrast medium. Therefore, selective catheterization of the bronchial artery was stopped. Subsequently, she was admitted to the intensive care unit for close monitoring. The patient developed further hemoptysis with acute worsening of oxygenation.
Surgery was deemed the procedure of choice in the treatment of massive recurrent hemoptysis. The patient underwent left upper lobectomy, and histopathologic examination of the resected specimen showed intra-alveolar hemorrhage with vascular congestion. The operation and the postoperative period were uneventful and the patient was discharged from the hospital after 5 days in good clinical condition. She has been well since then without any hemoptysis.
Systemic arterial supply from the descending thoracic aorta to the basal segment of the left lower lobe without a pulmonary artery supply is a rare congenital anomaly within the spectrum of the pulmonary sequestration. Bronchial arteries that originate outside the area between the T5 and T6 vertebrae at the level of the major bronchi are considered anomalous or aberrant4. The reported prevalence with an anomalous origin ranges from 8.3% to 35%1. As a general trend, the anomalous artery arising from the descending thoracic aorta supplies the left lower lobe, and the one arising from the abdominal aorta (or the celiac axis) supplies the right lower lobe, with normal pulmonary arterial branches as well.
In our case, an anomalous systemic artery arising from the des cending thoracic aorta fed the lingular segment of the left upper lobe. This type of arterial configuration through the interlobar fissure into the upper lobe is extremely rare among previous reports of anomalous systemic arterial supply to the lung.
Because it has normal bronchial connections and the involved lung is not sequestrated, this arterial anomaly is now considered to be different from true sequestration. Some synonyms for this clinical entity, such as "arterial pulmonary malinosculation" and "systemic arterializations of lung without sequestration," have been used recently, but the most widely accepted term is "anomalous systemic arterial supply" (to the basal segments of the lung)5.
This aberrant bronchial artery configuration can be distinguished anatomically and angiographically from nonbronchial systemic collateral vessels, in that they extend along the course of the major bronchi6. It is essential that a normal airway is confirmed to make a diagnosis of this anomaly.
The pathophysiology of these anomalies was understood from the embryology of the lung. Flisak et al.7 suggested that, except in the setting of pleural adhesions, pulmonary oligemia is a constant underlying factor either with persistence or reestablishment of pulmonary-splanchnic collaterals. The most likely theory is that if the early branches arising from the aorta persist, this causes one type of anomalous systemic supply to the lung.
Selective pulmonary and aortic angiography is essential in confirming the diagnosis and in planning the operation when surgical management is necessary. To accurately evaluate the vascular anatomy and to plan definitive surgical therapy, it is necessary to know whether normal pulmonary arteries branch to the involved lung, or whether the aberrant artery provides the blood flow to the entire involved lung with capillary phase or demonstrates direct drainage of the blood into the pulmonary vein.
Recently, multiple detector computed tomography (MDCT) or MDCTA has largely replaced angiography. MDCT provides a more precise depiction of bronchial, nonbronchial systemic, and pulmonary arteries than does conventional angiography in patients undergoing endovascular treatment for hemoptysis89. MDCTA has become more widely used for evaluating the anatomy of bronchial arteries, and bronchial arteries of high origin now are identified more frequently. In adults, normal bronchial arteries measure less than 1.5 mm in diameter at their origin and 0.5 mm at their point of entry into a bronchopulmonary segment10. A bronchial artery larger than 2 mm at computed tomography is most likely abnormal11. Hypertrophic bronchial arteries are easily visualized as enhancing nodular or tubular structures within the mediastinum and around the central airway on computed tomographic scans12.
In our case, MDCTA revealed an anomalous left bronchial artery from distal thoracic aorta to left upper lobes with an intrapulmonary course along the left main bronchus and lower lobar bronchus.
Although most of the patients with the anomaly are usually asymptomatic and detection of the condition may be done incidentally on radiographs, the main clinical symptoms of this disorder are hemoptysis and exertional dyspnea713. The shunt, when sufficiently large, produced exertional dyspnea, that was resulted from left-sided cardiac overload and congestive heart failure.
In past decades, bronchial artery embolization has become an established procedure in the management of massive and recurrent hemoptysis14. However, aberrant bronchial arteries can cause technical challenges when identifying a bleeding artery during endovascular treatment for the management of hemoptysis, thus necessitating surgery15.
The improvements in materials and applied techniques, as well as reduction in the duration of the procedure with latestgeneration digital equipment, make successful embolization increasingly attainable6. Moreover, surgical procedure is indicated only to reduce symptoms such as hemoptysis or dyspnea on exertion.
In most reported cases, lobectomy/segmentectomy is the standard treatment for such patients2. Other operational procedures include segmentectomy, anastomosis between the anomalous artery and pulmonary artery, and ligation of the anomalous artery.
In the surgical management of anomalous systemic arterial supply to the lung, a rigorous preoperative evaluation of the perfusion area of the anomalous artery is crucial to delineate the appropriate anatomic components of the lung that should be removed.
In our patient, preoperative arteriography showed that the anomalous and tortuous artery fed only the lingular segments of the upper lobe; therefore, resection of the involved lobe was indicated. We performed left upper lobectomy without ligation of the origin of the aberrant artery from the descending thoracic aorta.
In this report, we describe the extremely rare case report about anomalous systemic artery to the lung arising from the descending thoracic aorta and feeding the lingular segment of the left upper lobe.
Figures and Tables
Figure 1
Selective bronchial angiograms reveal a hypertrophied bronchial artery (A) and vascular staining (B) in the apico-posterior segment of the left upper lobe.
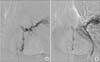
Figure 2
Anomalous systemic arterial supply to normal lung parenchyma in a 29-year-old woman with recurrent hemoptysis. Multidetector computed tomographic angiography in mediastinal window settings (A-C) shows multiple nodular and curvilinear structures with high attenuation within the mediastinal soft tissue (arrows in A-C) and left upper lobe that indicate systemic arteries. In lung window settings (D-F), scans showed hypertrophied vessels in the left upper lobe and ground-glass attenuation in the left upper lobe and left lower lobe that is probably due to hyperemia from systemic supply to the lung and aspirated blood.
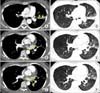
Figure 3
A coronal computed tomography (CT) image (A) shows aberrant bronchial artery (arrows in A) supplying the left upper lobe. After identifying aberrant bronchial artery in the CT and bronchial angiogram, bonchial artery embolization was performed to control hemoptysis in this patient. A coronal (B) and a volume-rendered CT image (C) reveal embolization coils (arrows in B and C) at the proximal portion of aberrant bronchial artery.

References
1. Cauldwell EW, Siekert RG. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet. 1948; 86:395–412.
2. Hiramatsu M, Iwashita M, Inagaki T, Matsudaira H, Hirano J, Odaka M, et al. Anomalous systemic arterial supply to separate lingular and basal segments of the lung: an anatomic consideration. Ann Thorac Surg. 2009; 88:1358–1360.
3. Albertini A, Dell'Amore A, Tripodi A, Del Giglio M, Pagliaro M, Calvi S, et al. Anomalous systemic arterial supply to the left lung base without sequestration. Heart Lung Circ. 2008; 17:505–507.
4. Cohen AM, Antoun BW, Stern RC. Left thyrocervical trunk bronchial artery supplying right lung: source of recurrent hemoptysis in cystic fibrosis. AJR Am J Roentgenol. 1992; 158:1131–1133.
5. Sade RM, Clouse M, Ellis FH Jr. The spectrum of pulmonary sequestration. Ann Thorac Surg. 1974; 18:644–658.
6. Sancho C, Escalante E, Dominguez J, Vidal J, Lopez E, Valldeperas J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol. 1998; 21:300–304.
7. Flisak ME, Chandrasekar AJ, Marsan RE, Ali MM. Systemic arterialization of lung without sequestration. AJR Am J Roentgenol. 1982; 138:751–753.
8. Battal B, Akgun V, Karaman B, Bozlar U, Tasar M. Normal anatomical features and variations of bronchial arteries: an analysis with 64-detector-row computed tomographic angiography. J Comput Assist Tomogr. 2011; 35:253–259.
9. Khalil A, Parrot A, Nedelcu C, Fartoukh M, Marsault C, Carette MF. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest. 2008; 133:212–219.
10. Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987; 135:463–481.
11. Osiro S, Wear C, Hudson R, Ma XX, Zurada A, Michalak M, et al. A friend to the airways: a review of the emerging clinical importance of the bronchial arterial circulation. Surg Radiol Anat. 2012; 34:791–798.
12. Do KH, Goo JM, Im JG, Kim KW, Chung JW, Park JH. Systemic arterial supply to the lungs in adults: spiral CT findings. Radiographics. 2001; 21:387–402.
13. Tao CW, Chen CH, Yuen KH, Huang MH, Li WY, Perng RP. Anomalous systemic arterial supply to normal basilar segments of the lower lobe of the left lung. Chest. 1992; 102:1583–1585.
14. Jiang S, Sun XW, Yu D, Jie B. Endovascular embolization of bronchial artery originating from the upper portion of aortic arch in patients with massive hemoptysis. Cardiovasc Intervent Radiol. 2014; 37:94–100.
15. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002; 22:1395–1409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download