Abstract
Although influenza A (H1N1) virus leads to self-limiting illness, co-infection with bacteria may result in cases of severe respiratory failure due to inflammation and necrosis of intra-airway, as pseudomembranous tracheobronchitis. Pseudomembranous tracheobronchitis is usually developed in immunocompromised patients, but it can also occur in immunocompetent patients on a very rare basis. We report a case of pseudomembranous tracheobronchitis complicated by co-infection of inflenaza A and Staphylococcus aureus, causing acute respiratory failure in immunocompetent patients.
In 2009, influenza A (H1N1) virus emerging from the Mexico and the United States spread worldwide, causing the World Health Organization to raise its pandemic alert1. South Korea was also affected by the pandemic from July 20092. Mild respiratory symptoms are common in influenza A (H1N1) infection. However, sometimes serious complications including severe pneumonia or acute respiratory distress syndrome (ARDS) still occur in some patients. Although influenza virus can cause pulmonary infiltration or hypoxemia by direct damage to respiratory epithelial cell, it is known that secondary pneumonia or bacterial coinfection is major factor affecting mortality or prognosis. Furthermore, the viral-bacterial interaction is considered as an important pathogenesis of respiratory complications in patient with influenza and bacterial coinfection34. Influenza can not only cause acute bronchitis but also worsen chronic respiratory disease including chronic obstructive pulmonary disease or asthma through invading the lower respiratory tract5. However, especially in immunocompetent patient, pseudomembranous tracheobronchitis associated with influenza have been very rare. We report a case of severe pseudomembranous tracheobronchitis complicated by H1N1 influenza.
A 42-year-old woman was admitted with dyspnea and enduring cough for several days. Although she had not suffered from any underlying illness, she had administered oseltamivir for influenza A (H1N1) which had been diagnosed using polymerase chain reaction (PCR) in a local clinic 4 days ago. When she was admitted, her vital signs were as follows: blood pressure, 90/60 mm Hg; pulse rate, 100 beats per minute; respiratory rate, 22 breaths per minute; and body temperature, 36.3℃. Oxygen saturation was 93.3% with 2 L/min of oxygen via nasal cannula. On auscultation of her chest, coarse breathing sounds were noted in both lung fields. Chest X-ray showed peribronchial consolidations, and multifocal ground glass opacities in both hilar areas (Figure 1). Laboratory findings revealed hypoxemia on the arterial blood gas analysis (pH, 7.390; PCO2, 27.9 mm Hg; PO2, 67.9 mm Hg; bicarbonate, 17.0 mmol/L), hemoglobin of 13.8 g/dL, white blood cell count of 4,640 cells/mm3 (neutrophils, 77.4%), and platelet count of 161,000 cells/mm3. Other findings showed C-reactive protein of 30.24 mg/dL, serum albumin of 3.7 g/dL, aspartate aminotransferase of 35 U/L , alanine aminotransferase of 27 U/L, total bilirubin of 0.9 mg/dL, and serum creatinine of 1.8 mg/dL. Chest computed tomography (CT) presented tracheobronchial wall thickening, multifocal patchy consolidations and nodular opacities with cavitations on both lungs (Figure 2). Bronchoscopy showed severe mucosal inflammation with sloughing and diffuse cobblestone-like multiple mucus swelling of exudates in whole bronchial tree, causing partial obstruction of airways, consistent with pseudomembranous tracheobronchitis (Figure 3). We couldn't perform bronchoscopic biopsy because she was severely desaturated during the procedure. After bronchoscopy, respiratory failure occurred and she was transferred to the intensive care unit to start the mechanical ventilation. Methicillin-sensitive Staphylococcus aureus (MSSA) was isolated from bronchial washing fluid. Acid fast bacillus stain and PCR for Mycobacterium tuberculosis were negative. Serum galactomannan test was negative. With the diagnosis of the pseudomembranous tracheobronchitis following influenza, we changed the antibiotics to ciprofloxacin and amoxicillin/clavulanate against MSSA. D uring mechanical ventilation, she required tube thoracotomy for bilateral pneumothoraces. In addition, infected pneumatoceles occurred, so that we drained the fluid from infected pneumatocele using a pigtail catheter and changed antibiotics to colistin against multi-drug resistant Acinetobacter baumannii which was isolated from the fluid in infected pneumatocele. After improvement of dyspnea and radiologic findings of chest X-ray, we could remove the pigtail catheter (Figure 4). The follow-up bronchoscopy showed much improvement (Figure 5), and she was discharged to home on 52nd hospital day.
Etiologies of pseudomembranous tracheobronchitis could be divided into infectious and non-infectious origin. Infectious causes are Corynebacterium, Aspergillus, Streptococcus, Bacillus cereus, Staphylococcus, and respiratory virus. Non-infectious causes are inflammatory bowel disease, endotracheal intubation, and post-transplantation6. Most of pseudomembranous tracheobronchitis is usually developed in patients with immunocompromised status, like as hematologic malignancy or transplantation, or long-term endotracheal intubation. However, pseudomembranous tracheobronchitis complicated by influenza in immunocompetent patient is very rare and could be fatal7.
The clinical feature of influenza A (H1N1) generally presented acute onset of respiratory illness which may result in pneumonia8. In pathogenesis of coinfection, influenza develops respiratory epithelial cell dysfunction which result in apoptosis of cell, and viral neuraminidase of influenza cleaves sialic acids in respiratory epithelial cell so that bacterial adhesion and dissemination could occur. Furthermore, the PBI-F2, a proapoptotic protein of influenza, enhances susceptibility to bacterial infection9. Especially, coinfection with Staphylococcus aureus could develop inflammation in trachea and bronchus. From the previous report that protease from S. aureus enhance influenza infectivity and proteolytic activity of hemagglutinin in mice10, we could suggest that coinfection with influenza and S. aureus might develop pseudomembranous tracheobronchitis.
Although radiologic finding doesn't show severe pneumonic infiltration, severe dyspnea and chocking can be occurred by influenza infection when the pseudomembranous tracheobronchitis causes the central airway obstruction adding the gas exchange disturbance from pneumonic infiltration1112. Therefore, when patient with influenza presents unexplained severe dyspnea inconsistent with radiologic finding, the pseudomembranous tracheobronchitis should be included in differential diagnosis.
Early empirical antiviral treatment with oseltamivir or zanamivir and antibiotic treatment with a respiratory fluoroquinolone or combination of beta-lactam and macrolide should be administered in all patients who suspect coinfection. In particular, when a patient with influenza presents signs of necrotizing pneumonia or sepsis including hemoptysis, pleural effusion, ARDS or leukopenia, empirical regimen using vancomycin or linezolid should be considered against the methicillin-resistant S. aureus. After the empirical management, it is recommended that antibiotics should be changed for specific organism isolated from blood or high-quality respiratory specimen. Despite of early empirical antiviral and antibiotic treatment, similar to ours, pseudomembranous tracheobronchitis accompanying progressive hypoxia may require supportive mechanical ventilation9.
In conclusion, the pseudomembranous tracheobronchitis with influenza infection is high risk for bacterial pneumonia and critical illness resulting in severe dyspnea and acute respiratory failure. When the patient with influenza undergo progressive hypoxemia and dyspnea notwithstanding empirical antiviral and antibiotic treatment, it is important that clinician should evaluate airway using CT or bronchoscopy.
Figures and Tables
 | Figure 1Plain chest radiograph presents peribronchial consolidations and multifocal ground glass opacities in both the hilar areas. |
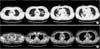 | Figure 2(A, B) Chest computed tomography reveals diffuse tracheobronchial wall thickening, multifocal patchy consolidations and nodular opacities with cavitations on both the lungs. |
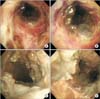 | Figure 3(A-D) Bronchoscopy shows severe mucosal inflammation with sloughing and diffuse cobblestone-like multiple swelling of exudates in whole bronchial tree, causing partial obstruction of airways, consistent with pseudomembranous tracheobronchitis. |
References
1. World Headth Organization. World now at the start of 2009 influenza pandemic [Internet]. Geneva: World Health Organization;2009. cited 2015 Mar 1. Available from: http://who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
2. Korea Centers for Disease Control and Prevention. H1N1 influenza A (H1N1) press release [Internet]. Seoul: Korea Centers for Disease Control and Prevention;2009. cited 2015 Mar 1. Available from: http://www.cdc.go.kr/.
3. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006; 19:571–582.
4. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006; 6:303–312.
5. Smith CB, Kanner RE, Golden CA, Klauber MR, Renzetti AD Jr. Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J Infect Dis. 1980; 141:271–280.
6. Talwar A, Patel N, Omonuwa K, Lisker G. Postintubation obstructive pseudomembrane. J Bronchol. 2008; 15:110–112.
7. Nam KH, Hong JS, Hong MY, Lim JM, Kim MH, Jung BH, et al. A case of pseudomembranous tracheobronchitis complicated by coinfection of 2009 pandemic influenza A/H1N1 and Staphylococcus aureus. Infect Chemother. 2011; 43:425–428.
8. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009; 361:1935–1944.
9. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013; 309:275–282.
10. Tashiro M, Ciborowski P, Klenk HD, Pulverer G, Rott R. Role of Staphylococcus protease in the development of influenza pneumonia. Nature. 1987; 325:536–537.
11. Namba Y, Mihara N, Tanaka M. Fulminant tracheobronchitis caused by methicillin-resistant Staphylococcus aureus (MRSA). Nihon Kyobu Shikkan Gakkai Zasshi. 1997; 35:969–973.
12. Herrmann RE, Ogura GI, Johnson ES, Toll HW Jr, White WC. Respiratory deaths associated with Asian influenza epidemic: report of twenty-three cases. J Am Med Assoc. 1958; 166:467–471.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download