Abstract
Malignant pleural mesothelioma (MPM) is an aggressive, treatment-resistant, and generally fatal disease. A 68-year-old male who was diagnosed with MPM at another hospital came to our hospital with dyspnea. We advised him to take combination chemotherapy but he refused to take the treatment. That was because he had already received chemotherapy with supportive care at another hospital but his condition worsened. Thus, we recommended photodynamic therapy (PDT) to deal with the dyspnea and MPM. After PDT, the dyspnea improved and the patient then decided to take the combination chemotherapy. Our patient received chemotherapy using pemetrexed/cisplatin. Afterwards, he received a single PDT treatment and then later took chemotherapy using gemcitabine/cisplatin. The patient showed a survival time of 27 months, which is longer than median survival time in advanced MPM patients. Further research and clinical trials are needed to demonstrate any synergistic effect between the combination chemotherapy and PDT.
Malignant mesothelioma develops from mesothelial cells. Malignant mesothelioma known as pleural tumor arises mostly in the pleura. But it develops into neoplasm in other body parts since mesothelial cells are present in peritoneum, tunica vaginalis testis and pericardium beside pleura. In fact, it is known that 70% of malignant mesothelioma appears in the pleura, less than 30% in the peritoneum, and rare in the other body parts1. Malignant pleural mesothelioma (MPM) is a fatal disease with poor prognosis and median survival time of 6 to 17 months2. Single therapy is not effective. Treatments range from radical resection, radiation and chemotherapy to pleurodesis which is performed while inserting chest-tube for control pleural effusion. For advanced MPM, pemetrexed/cisplatin combination chemotherapy is considered as the first line of the standard treatment. Treatment options are limited for the advanced MPM that does not respond to the combination chemotherapy. For this case, new approach like photodynamic therapy (PDT) is being tried.
PDT is a method of cancer treatments. It involves abundant oxygen inside the body, light source of laser and light-sensitive photosensitizers3,4. Research and treatment for MPM by using PDT started in the 1990s5, and it is now being used in clinical trials.
In this respect, along with reviewing available literature, we report the first Korean case of PDT for a patient with advanced MPM showing longer survival time than the average median survival time.
A 68-year-old male came to our hospital because of dyspnea. He had symptoms like dyspnea and weight loss for 4 months before he came to our hospital. He was admitted to another university hospital and diagnosed with MPM through thoracoscopic biopsy. So, pathological examination was not performed on the patient in our hospital. In the other hospital, chemotherapy had been already performed on him but the symptoms did not improve. To control dyspnea, pleurodesis was carried out following two times of chest tube insertion. Instead, increased effusion on the right sided pleura aggravated dyspnea. Eventually he had to come to our hospital with aggravated dyspnea. He had taken medications for 3 years to treat hypertension. His smoking history was 30 pack years. His job had nothing to do with asbestos. At the time of admission in our hospital, his initial vital signs are as follows: blood pressure, 150/80 mm Hg; respiratory rate, 28 breaths per minute; pulse rate, 86 beats per minute; and body temperature, 36.5℃. Chest auscultation revealed decrease in breathing sound over the entire right lung. The chest radiography taken at the time of admission showed about 11-cm mass with multiple pleural nodules and pleural effusion in the right hemithorax (Figure 1). Chest computed tomography (CT) scans revealed about 11.5×16-cm mass with pleural effusion and disseminated pleura metastasis in the right hemithorax. Direct invasion to paratracheal lymph node, right paraaortic nodal station, and pleura in the back of the right lower lobe was observed (Figure 2A, B). Bone scan revealed bone metastasis on the ninth right rib and the fourth lumbar spine.
The patient with severe dyspnea did not respond to oxygen supply. He had already received chemotherapy in the other hospital but his symptom aggravated. He was disappointed with the treatment and he refused to take any more chemotherapy. So we advised him to try photodynamic therapy (PDT) for treatment and he took the advice.
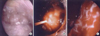
To perform PDT, photosensitizer used for the patient is photogem (Lomonosov Institute of Fine Chemical, Moscow, Russia), and 2.5 mg/kg was injected through intravenous 48 hours before the procedure. To prevent complications due to skin photosensitive reaction, the patient was advised to avoid direct light for 48 hours. After that, under general anesthesia the patient had thoracoscope inserted after posterolateral thoracotomy. As a light delivery device, flexible cylindrical fiber was used, and diode laser system (Biolitec, Jena, Germany) was employed based on 632 nm wavelength, with 15 J/cm2 light doses being applied (Figure 3A-C). Following PDT, light shield was carried out to avoid sun light. After PDT, dyspnea improved and complications were not observed. Once his symptom and general condition got better, he changed his mind and decided receive chemotherapy.
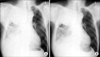
Pemetrexed and cisplatin combination chemotherapy was performed. Pemetrexed 500 mg/m2 with cisplatin 60 mg/m2 were administered once every 3 weeks. The patient was discharged with better condition of dyspnea (Figure 4A, B). After six cycles of pemetrexed/cisplatin combination chemotherapy, the tumor volume had not shown significant interval change. But, he remained relatively stable for 6 months after multimodality therapy. At 7 months of the multimodality treatment, dyspnea was aggravated and progression of disease was shown on the chest CT. So the PDT single treatment was carried out in the same way and the symptom improved. Although significant decreased in the mass volume was not seen, his symptom improved and he remained stable without further treatment during the 9 months after the second PDT. Ten months after the second PDT, dyspnea became worse again, and progression of disease was shown on the chest CT. The third PDT was intended but failed to be performed due to the patient's financial reason. Instead, the patient took combination chemotherapy. The patient was treated with gemcitabine 1,000 mg/m2 on days 1 and 8, and cisplatin 60 mg/m2 on day 1. This chemotherapy regimen was repeated once every 3 weeks. However dramatic response was not shown. He died 27 months after initial diagnosis with MPM.
MPM is a rare disease. For the United States, each year, about 2,500 patients are reported and it happens to more men than women. According to the report by Korea Central Cancer Registry, 111 patients of both men and women were registered in 2011, which is 0.1% of total cancer patients. The connection between exposure to asbestos and the development of mesothelioma has proved strong through studies since the first report of 33 cases by Wagner et al. in 19602. For some cases, it is known to appear without exposure to asbestos. The most common symptoms include chest pain caused by involving the rib and thoracic cavity, and dyspnea associated with chest compression and pleural effusion2. For diagnosis of MPM, chest radiography and chest CT based on clinical symptoms are performed in order to see the existence of the tumor, its condition and the location. MPM is confirmed through pathologic biopsy1,2,6. Staging is to assign as I-IV in accordance with TNM-based staging system recommended by the International Mesothelioma Interest Group (IMIG). It is known for poor prognosis with median survival time of 1 year or so2. Despite traditionally multimodality therapy, advanced MPM is a fatal disease with median survival time of less than one year. Poor prognosis and recurrence after the treatment are key problems with the disease, thus the biggest interest lies in developing a topical treatment7,8. In this respect, PDT as a topical treatment increasingly draws attention.
The mechanism of PDT is as follows. Photosensitizer is fast absorbed into body cells through blood vessels. Non-cancer cells excrete the photosensitizer fast out of body, but cancer cells are slow. As a result, the photosensitizer remains selectively on the cancer cells after intravenously injection. Photosensitizer is inert until activated by the light of laser with a specific wavelength. Direct light delivery is obtained by placement of optic fibers at the tumor site. Light activation of the photosensitizer lead to the production of reactive oxygen species and direct tumor cell death4,9,10. The procedure of PDT is relatively easy with less risky of hemorrhage and perforation, and its side effects are minimal since photosensitizer injected for treatment works selectively on target cells. Unlike cumulative toxic chemotherapy, PDT is less toxic and does not create complications due to the injury around the lesion unlike radiation therapy. It has advantage of performing several times on the same lesion without resistance. As a complication, skin photosensitivity can happen due to the side effect of photosensitizers. Patients may become hypersensitive to light when injected photosensitizers, and show various symptoms like erythema, blister, pain and swelling. However once they follow a simple guideline to avoid sun light, side effects are not serious and mostly temporary. A literature review shows clinical trials treating 40 patients with MPM based on this PDT mechanism. The study targeting patients treated with surgery and PDT compares survival rate for 2 years based on staging. Sixty-one percentage of patients with stage I-II MPM survived 2 years and those with stage III-IV MPM shows 0% of the survival rate11. Another study of patients with III-IV stage MPM compares patients given existing treatment plus PDT and those without PDT. Both groups do not show any meaningful difference in the median survival time12. With the development of new photosensitizers and improved laser technique, it is expected to see more attention to PDT and improved results of treating MPM with the therapy. PDT can be combined with lung-sparing pleurectomy, decortication and other treatments such as adjuvant chemotherapy and/or radiation therapy13. Therefore, PDT could be suggested as a way of adjuvant treatment for MPM.
Traditionally used treatments in MPM include supportive care for relieving chest pain or dyspnea, and surgery, radiation therapy, as well as chemotherapy. Surgery can be applied to the stage I and II. Surgery by itself does not improve survival rate and so it is better to combine with radiation therapy or chemotherapy. Radiation therapy is partially used for chest pain caused by metastasis or adjunctively used in combination with other approaches14. Chemotherapy is commonly used for advanced MPM. Single chemotherapy is reported to show less than 20% of response rate but combination chemotherapy with cisplatin is reported to increase the response rate to 27%-48%1,2. Another study shows combination therapy of pemetrexed and cisplatin increases median survival, delays progression and improves response rate compared to pemetrexed alone or cisplatin alone therapy15. Thus, currently combination chemotherapy with pemetrexed/cisplatin is widely recommended as the first line treatment for MPM.
In this case, our patient refused to take combination chemotherapy with pemetrexed/cisplatin, initially. So we performed PDT as first line treatment for MPM. After improved of dyspnea, we carried out the pemetrexed/cisplatin combination chemotherapy. This case shows that a patient with advanced MPM remained stable for 9 months after PDT single treatment. In short, it is good idea to consider PDT to deal with advanced MPM patients who do not show response to the existing combination chemotherapy but the symptom aggravate.
In summary, MPM is a rare case of malignant disease present on the pleura with poor prognosis. Existing operation, chemotherapy, and radiation therapy have been performed a lot, but it is still hard to treat advanced MPM. The patient of our case with advanced MPM showed 27 months of survival time after we treated him with the combination chemotherapy and PDT. Consequently, we suggest PDT combined with other existing treatments could be applied as an option for advanced MPM cases to improve patient's symptoms and extend survival time. It is thought to decide the role of PDT in treating MPM through lots of research in near future.
Figures and Tables
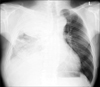 | Figure 1The initial chest radiograph at our hospital shows a huge mass with multiple pleural nodules and pleural effusion in the right hemithorax. |
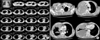 | Figure 2(A) The initial chest computed tomography (CT) scans at another hospital shows a pleural mass without pleural effusion in the right upper chest. (B) After 4 months, the initial chest CT scans at our hospital shows a mass of about 11.5×16 cm with pleural effusion and disseminated pleura metastasis in the right hemithorax. Direct invasion to paratracheal lymph node, right paraaortic nodal station, and pleura in the back of the right lower lobe was observed. |
References
1. Ahmed I, Ahmed Tipu S, Ishtiaq S. Malignant mesothelioma. Pak J Med Sci. 2013; 29:1433–1438.
2. Pistolesi M, Rusthoven J. Malignant pleural mesothelioma: update, current management, and newer therapeutic strategies. Chest. 2004; 126:1318–1329.
3. Regillo CD. Update on photodynamic therapy. Curr Opin Ophthalmol. 2000; 11:166–170.
4. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61:250–281.
5. Takita H, Mang TS, Loewen GM, Antkowiak JG, Raghavan D, Grajek JR, et al. Operation and intracavitary photodynamic therapy for malignant pleural mesothelioma: a phase II study. Ann Thorac Surg. 1994; 58:995–998.
6. Rodriguez E, Baas P, Friedberg JS. Innovative therapies: photodynamic therapy. Thorac Surg Clin. 2004; 14:557–566.
7. Schouwink H, Rutgers ET, van der Sijp J, Oppelaar H, van Zandwijk N, van Veen R, et al. Intraoperative photodynamic therapy after pleuropneumonectomy in patients with malignant pleural mesothelioma: dose finding and toxicity results. Chest. 2001; 120:1167–1174.
8. Su S. Mesothelioma: path to multimodality treatment. Semin Thorac Cardiovasc Surg. 2009; 21:125–131.
9. Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res. 2010; 46:216–226.
10. Friedberg JS. Radical pleurectomy and photodynamic therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012; 1:472–480.
11. Moskal TL, Dougherty TJ, Urschel JD, Antkowiak JG, Regal AM, Driscoll DL, et al. Operation and photodynamic therapy for pleural mesothelioma: 6-year follow-up. Ann Thorac Surg. 1998; 66:1128–1133.
12. Pass HI, Temeck BK, Kranda K, Thomas G, Russo A, Smith P, et al. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol. 1997; 4:628–633.
13. Friedberg JS. Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009; 21:177–187.
14. Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg. 2001; 25:210–217.
15. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003; 21:2636–2644.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download