Abstract
Pneumothorax is an extremely rare complication of non-tuberculous mycobacterial infection. A 52-year-old man presenting with difficulty breathing and chest pain was admitted to our hospital. A right-sided pneumothorax was observed on chest radiography and chest computed tomography showed multiple cavitating and non-cavitating nodules with consolidation in the upper to middle lung zones bilaterally. Serial sputum cultures were positive for Mycobacterium kansasii, and he was diagnosed with pulmonary M. kansasii disease complicated by tension pneumothorax. After initiation of treatment including decortications and pleurodesis, the patient made a full recovery. We herein describe this patient's course in detail and review the current relevant literature.
The rates of non-tuberculous mycobacterial (NTM) isolation from sputum specimens and the incidence of pulmonary NTM disease have been increasing worldwide1. In particular, Mycobacterium kansasii is one of the most common causative pathogens of pulmonary NTM worldwide2. In Korea, however, M. kansasii is a relatively uncommon cause of pulmonary NTM disease, and constitutes about 2%-4% of NTM organisms detected in all specimens3.
The clinical course of M. kansasii lung disease is quite similar to that of M. tuberculosis2. Additionally, the chest radiographic findings resemble those of reactivation pulmonary tuberculosis (TB) including cavitary infiltrates with an upper lobe predilection or nodular and bronchiectatic lung disease4. Therefore, pulmonary M. kansasii disease is often indistinguishable from pulmonary TB on initial presentation.
Although pneumothorax in patients with pulmonary TB is a well-known complication, pneumothorax secondary to pulmonary NTM disease is rare5. Few studies have reported the clinical features of pneumothorax in patients with pulmonary NTM disease, and those have focused primarily on pulmonary M. avium complex (MAC) disease56. We report a case of pulmonary M. kansasii disease that successfully treated through medical and surgical management, although complicated by tension pneumothorax.
A 52-year-old man was admitted to our hospital with acute onset of difficulty breathing and chest pain. On the initial physical examination, the patient appeared to be acutely ill. He had no significant past medical history including lung disease. Laboratory studies, however, revealed that the patient had undiagnosed diabetes mellitus with a random glucose level of 398 mg/dL and a hemoglobin A1c of 9.9%. He was also an active smoker with a two pack-per-day smoking history of 30 years' duration. Height and weight were 179.7 cm and 81.1 kg, respectively. His body mass index was 25.1 m2/kg, which was classified as mildly overweight. His vital signs were as follows: blood pressure, 102/74 mm Hg; pulse rate, 110 beats per minute; respiratory rate, 24 per minute; and body temperature, 37.2℃. Auscultation revealed decreased breath sounds in the right lower lung field. Arterial blood gas analysis on room air showed a mixed metabolic and respiratory acidosis with severe hypoxemia: pH of 7.327, PaCO2 of 32.6 mm Hg, PaO2 of 58.2 mm Hg, and SaO2 of 88.2%. Chest radiography revealed a large hyperlucency in the right lung field (Figure 1A). A diagnosis of right-sided tension pneumothorax was made, and a 24-French chest tube was placed and connected to a water seal device, thereby re-expanding the right lung (Figure 1B). A chest computed tomography revealed multiple cavitating and non-cavitating nodules with consolidations in the upper to middle lung zones bilaterally, as well as extensive subcutaneous emphysema in the right chest wall and a small amount of residual hydropneumothorax of the right lung (Figure 2). The sputum smear was positive for acid-fast bacilli, and sputum polymerase chain reaction (PCR) was negative for both Mycobacterium tuberculosis and NTM. We initially considered a diagnosis of pulmonary TB, and anti-TB medications including isoniazid 300 mg, rifampicin 600 mg, ethambutol 1,200 mg, and pyrazinamide 1,500 mg were started. We hypothesized that his tension pneumothorax had likely been caused by a ruptured cavitary lesion arising from M. tuberculosis infection.
One month after admission, M. kansasii was detected on each of three consecutive sputum cultures. Table 1 shows the drug susceptibility pattern of the M. kansasii isolate. We ultimately diagnosed him with pulmonary NTM disease complicated by tension pneumothorax caused by M. kansasii. Standard medical therapy for pulmonary M. kansasii disease was maintained, and the diffuse lung infiltration seen on chest radiography resolved. However, despite these improvements, prolonged air leakage from the chest tube was noted and inflation of the lung was incomplete. A flexible bronchoscopy was subsequently performed, though no endobronchial lesions were identified. Despite several interventions such as increasing the negative pressure through the chest tube and chemical pleurodesis using an autologous blood patch and doxycycline, air leakage from the chest tube persisted and the right lung was noted to not fully expand on chest radiography (Figure 1C). Decortications and pleurodesis via video-assisted thoracoscopic surgery were the next step in management of this patient, after which the air leak resolved and the chest tube was removed. Follow-up sputum cultures after 6 months of treatment were negative for M. kansasii. Additionally, follow-up chest radiography after 18 months of treatment exhibited full lung expansion and showed only post-infectious sequelae without relapse (Figure 1D).
In a country with a significant TB burden, it is important to distinguish pulmonary NTM disease from pulmonary TB in clinical practice. Although previous reports have described clinical differences between pulmonary M. kansasii and pulmonary TB, it is very difficult to differentiate between these two diseases based on clinical evidence alone7. Among the notable differences, the M. kansasii group has been associated with a higher rate of chest pain (74% vs. 26%; p=0.0001), coexistent lung disease (60% vs. 30%; p=0.0001), and chronic obstructive pulmonary disease (COPD) (35% vs. 4%; p=0.0001) compared with M. tuberculosis7. The incidence of cavitation has been shown to be similar in both groups (56% vs. 69%; p=0.07). Additionally, in one study, while patients with M. kansasii showed no evidence of pleural effusion, 10% of the patients in the M. tuberculosis had pleural effusions7.
There have been several reports on the radiologic findings of pulmonary M. kansasii disease. While studies prior to 2000 have noted a high incidence (75%-96%) of cavitation in patients with pulmonary M. kansasii disease, recent studies have exhibited a relatively low detection rate (32%-57%) of cavitary lesions389. A retrospective study of 41 patients reported clinical and radiological features and treatment outcomes of patients with pulmonary M. kansasii disease3. Radiographic findings in these patients included nodules (n=22, 54%), consolidation (n=22, 54%), cavitation (n=13, 32%), and pleural effusion (n=3, 8%). Another retrospective study reported that pulmonary M. kansasii disease was associated with more cavitations (57% vs. 3%; p=0.001) and no pleural complications compared with pulmonary M. simiae disease8. In 2012, a study that examined radiological features of pulmonary M. kansasii disease (n=64) compared with other pulmonary NTM diseases (n=34) was performed9. This study demonstrated that pulmonary M. kansasii disease showed more cavitations (53% vs. 6%; p<0.001) though infiltrative lesions were not statistically different between groups (51% vs. 35%; p=0.410)9. But, few reports identify patients with pulmonary M. kansasii disease complicated by pneumothorax5.
Secondary pneumothorax in patients with pulmonary NTM disease is considered to be a rare complication, and the few case reports and case series that have been published are limited to an association with MAC610. Unlike pulmonary NTM disease, pulmonary TB is one of the most frequent causes of secondary pneumothorax in patients with COPD, and the incidence rate of pneumothorax in patients with pulmonary TB reportedly ranges from 0.6% to 1.4%11. Recently, a retrospective study of spontaneous pneumothorax and pulmonary TB found that during a 20-year follow-up period, 0.95% of 2,089 pulmonary TB cases presented with secondary pneumothorax and were treated with pleural drainage, usually with a favorable outcome11.
Few reports describing pneumothorax in patients with pulmonary NTM disease have been published56. And, these studies have mainly focused on disease caused by MAC56. A retrospective study of 18 cases of pulmonary MAC disease complicated by pneumothorax found that MAC was often difficult to treat and could recur easily6. The incidence of pneumothorax in patients with active pulmonary MAC disease was unexpectedly higher, and the calculated complication rate was 2.4%6. Predictive risk factors for pneumothorax included old age and advanced MAC disease6. Although our patient was a middle aged male without history of previous lung disease, advanced pulmonary M. kansasii disease was present. The other retrospective study of 220 patients with pulmonary NTM disease, nine patients (4.1%) were complicated with pneumothorax. The causative microorganisms were MAC in eight patients, and M. kansasii in only one patient5. A patient with pulmonary M. kansasii disease complicated by pneumothorax was an 85-year-old man, and had underlying COPD. In radiological findings, cavity was existed and extent of NTM was within the unilateral lung field5.
To our knowledge, there have not been reports that have focused on pulmonary M. kansasii disease as the causative microorganism of secondary pneumothorax. This is the first report to describe tension pneumothorax as a complication of pulmonary M. kansasii disease without prior lung diseases. Based on our experience with this patient, even if the clinical situation is strongly suggestive of pulmonary TB, it is necessary to identify the causative microorganism via culture for acid-fast bacilli or PCR for pulmonary NTM disease and pulmonary TB. In conclusion, patients presenting with pneumothorax and cavitary lung disease require further workup to distinguish pulmonary M. kansasii disease from pulmonary TB.
Figures and Tables
 | Figure 1(A) Chest radiography showed a right pneumothorax on admission. (B) The right lung was nearly expanded after chest tube placement. (C) The right lung that did not fully expand despite medications and pleural management. (D) Follow up chest radiography after 18 months of treatment revealed fully expanded right lung and showed post-infectious sequelae without relapse. |
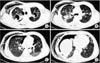 | Figure 2A chest computed tomography after chest tube placement revealed multiple cavitating and non-cavitating nodules with consolidations in the upper to middle lung zones bilaterally, with extensive subcutaneous emphysema in the right chest wall and a small amount of residual hydropneumothorax of the right lung (A-D). |
Acknowledgements
This research was supported by the 2015 scientific promotion program funded by Jeju National University.
References
1. Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis. 2010; 16:294–296.
2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
3. Park HK, Koh WJ, Shim TS, Kwon OJ. Clinical characteristics and treatment outcomes of Mycobacterium kansasii lung disease in Korea. Yonsei Med J. 2010; 51:552–556.
4. Griffith DE, Brown-Elliott BA, Wallace RJ Jr. Thrice-weekly clarithromycin-containing regimen for treatment of Mycobacterium kansasii lung disease: results of a preliminary study. Clin Infect Dis. 2003; 37:1178–1182.
5. Kobashi Y, Mouri K, Obase Y, Kato S, Oka M. Clinical analysis of patients with pulmonary nontuberculous mycobacterial disease complicated by pneumothorax. Intern Med. 2013; 52:2511–2515.
6. Hagiwara E, Komatsu S, Nishihira R, Shinohara T, Baba T, Ogura T. Clinical characteristics and prevalence of pneumothorax in patients with pulmonary Mycobacterium avium complex disease. J Infect Chemother. 2013; 19:588–592.
7. Shitrit D, Peled N, Bishara J, Priess R, Pitlik S, Samra Z, et al. Clinical and radiological features of Mycobacterium kansasii infection and Mycobacterium simiae infection. Respir Med. 2008; 102:1598–1603.
8. Matveychuk A, Fuks L, Priess R, Hahim I, Shitrit D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Respir Med. 2012; 106:1472–1477.
9. Kobashi Y, Fukuda M, Yoshida K, Miyashita N, Oka M. Pulmonary Mycobacterium intracellulare disease with a solitary pulmonary nodule detected at the onset of pneumothorax. J Infect Chemother. 2006; 12:203–206.
10. Asai K, Urabe N. Acute empyema with intractable pneumothorax associated with ruptured lung abscess caused by Mycobacterium avium. Gen Thorac Cardiovasc Surg. 2011; 59:443–446.
11. Freixinet JL, Caminero JA, Marchena J, Rodriguez PM, Casimiro JA, Hussein M. Spontaneous pneumothorax and tuberculosis: long-term follow-up. Eur Respir J. 2011; 38:126–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download