Abstract
Background
The efficacy of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy can be measured based on the rate of treatment response, based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria or progression-free survival (PFS). However, there are some patients harboring sensitive EGFR mutations who responded poorly to EGFR-TKI therapy. In addition, there is variability in the PFS after EGFR-TKI treatment.
Methods
We performed a retrospective analysis of the medical records of 85 patients with non-small cell lung cancer, who had achieved a stable disease or better response at the first evaluation of treatment response, after receiving a 2-month course of gefitinib. We calculated the tumor shrinkage rate (TSR) by measuring the longest and perpendicular diameter of the main mass on computed tomography before, and 2 months after, gefitinib therapy.
Results
There was a significant positive correlation between the TSR and PFS (R=0.373, p=0.010). In addition, a simple linear regression analysis showed that the TSR might be an indicator for the PFS (B±standard error, 244.54±66.79; p=0.001). On univariate analysis, the sex, histologic type, smoking history and the number of prior chemotherapy regimens, were significant prognostic factors. On multivariate regression analysis, both the TSR (β=0.257, p=0.029) and adenocarcinoma (β=0.323, p=0.005) were independent prognostic factors for PFS.
Non-small cell lung cancer (NSCLC) is a leading cancer-related death cause worldwide. It is well known that most patients are diagnosed with NSCLC at an incurable advanced stage. Over the past decade, there have been considerable advances in the treatment of patients with NSCLC.
To date, it has been shown that the epidermal growth factor receptor (EGFR) mutation status is closely associated with the efficacy of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy. Moreover, several studies (IPASS, NEJ002, WJTOG3405, and First Signal studies) have attempted to assess the efficacy of EGFR-TKI as the first-line of treatment as compared with the standard platinum doublet treatment in patients with EGFR mutations. This led to changes in the standard treatment regimen in patients with NSCLC with EGFR mutations 1234.
The efficacy of EGFR-TKI therapy can be measured based on the rate of treatment response according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria or progression-free survival (PFS). Despite the advantage of EGFR mutations (exon 19 del or L858R), however, there are some patients who poorly responded to EGFR-TKI therapy. In addition, there is variability in the PFS after EGFR-TKI treatment in patients with EGFR mutations. These findings indicate that the efficacy of EGFR-TKI therapy is also subject to other factors than the EGFR mutation status.
Recent studies have shown that the intratumoral heterogeneity with EGFR mutations is closely associated with the response and prognosis56. The intratumoral heterogeneity is referred to as the co-existence of both benign and malignant cells or mutant and wild-type EGFR within a single lesion. Michor and Polyak7 showed that clonal diversity also exists within malignant cells. This leads to the speculation that both well-responsive and poorly-responsive cells to EGFR-TKI therapy may coexist within a single lesion. Chen et al.5 conducted a study to compare the intratumoral heterogeneity with EGFR mutations between patients with NSCLC and those with adenocarcinoma with one or more additional lesions. These authors concluded that compare the intratumoral heterogeneity with EGFR mutations is linked to a mixed response to EGFR-TKI5. Taniguchi et al.6 collected surgical specimens from patients with recurrent NSCLC. The specimens were cut at a thickness of 35 µm, 50-60 areas of which contained cancer cells. These authors measured the percentage of EGFR gene mutation in each sample, thus showing that the PFS and overall survival (OS) were significantly shorter in patients with EGFR heterogeneity receiving gefitinib therapy6.
We introduced the tumor shrinkage rate (TSR) by measuring the longest diameter and perpendicular diameter of the main mass on computed tomography (CT) scans at baseline and 2 months after gefitinib therapy. We have speculated that the TSR might be a prognostic factor for PFS because it reflexes the ratio of cancer cells harboring sensitive mutation to EGFR-TKI.
We analyzed 90 patients with NSCLC who achieved a stable disease or better response at the first evaluation of treatment response after receiving a 2-month course of gefitinib therapy between August of 2005 and December of 2010 at Chungnam National University Hospital. The patient records and information were anonymized and de-identified before analysis. In our clinical series of patients, a diagnosis of NSCLC was made after histopathological examinations. In addition, we did not perform the EGFR mutation test to determine whether our clinical series of patients are indicated in gefitinib therapy. Inclusion criteria for the current study are as follows: (1) patients aged 18 years or older, (2) patients with Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2 and (3) patients who were continuously given gefitinib 250 mg once daily regimen until the disease progression. We excluded the following five cases: (1) patients with lost to follow-up (n=2) and (2) patients who discontinued taking gefitinib 250 mg once daily regimen due to adverse effects (n=3). The adverse effects include the Common Toxicity Criteria (CTC) grade 3 or 4 hepatotoxicity and grade 3 pneumonitis. We therefore enrolled a total of 85 patients (n=85). The current study was approved by the Institutional Review Board (IRB) of our medical institution (CNUH 2013-07-007). We performed a retrospective analysis of the medical records including radiology reports.
The treatment response was first evaluated at 2 months after gefitinib therapy. The tumor size was defined as the value of the longest diameter of the axial, coronal and sagittal sections of the main mass multiplied by the perpendicular diameter (Figure 1). Each diameter was measured using a picture archiving communication system supporting digital imaging and communications in medicine by two board-certified specialists in thoracic radiology. The main mass was defined as the lesion with the longest diameter which could be measured. Tumor size and TSR were calculated according to the following formula:
Tumor size=The longest diameter×Perpendicular diameter of main mass
TSR=(Tumor size before gefitinib therapy-Tumor size at 2 months after gefitinib therapy)/Tumor size before taking gefitinib
To determine the treatment response, we performed a CT scan at a 2-month interval based on the RECIST 1.1 criteria. The PFS was defined as the length of period elapsed since gefitinib was first administered to the patients until the disease progression was first observed. All the 85 patients received gefitinib until the disease progression. This indicates that the duration of gefitinib treatment is equal to the PFS.
To identify any correlation between TSR and PFS, we performed the Pearson correlation analysis. For univariate analysis, we performed the Student's t test. In addition, we also performed a linear regression analysis for multivariate analysis. Statistical analysis was done using the PASW statistics version 18.0 (IBM Co., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
The baseline and clinical characteristics are presented in Table 1. The mean TSR was 0.29±0.04 and the mean PFS was 276±24 days. A simple linear regression analysis showed that the TSR might be an indicator for the PFS (β±standard error, 244.54±66.79; p=0.001). There was a significant positive correlation between TSR and PFS (R=0.373, p=0.010) (Figure 2A). There was also a significant positive correlation between PFS and the sum of values those were calculated in all target lesions of RECIST 1.1 criteria (R=0.399, p=0.010) (Figure 2B). In addition, the response rate (%) calculated by the sum of diameters according to RECIST 1.1 criteria correlated with PFS (R=0.357, p=0.010) (Figure 2C).
We performed a univariate analysis of the correlation between baseline characteristics and PFS, thus showing that the sex, histologic type, smoking history and the number of prior chemotherapy regimens were significant prognostic factors. The PFS was significantly longer in female as compared with male (p=0.020). In addition, it was also significantly longer in patients with adenocarcinoma as compared with those with other histologic types of NSCLC (p=0.001). Moreover, it was also significantly longer in patients with no smoking history as compared with those with a smoking history (p=0.012). Furthermore, it was also significantly shorter in patients with two or more prior chemotherapy regimens (p=0.017) (Table 2). We also performed a multivariate analysis with the adjustment of such variables as TSR, histologic type, sex, age, ECOG performance status, smoking history, number of prior chemotherapy regimens and the best treatment response to prior chemotherapy in patients (n=79) who were able to be reviewed on medical records. Thus, we found that both TSR (β=0.257, p=0.029) and adenocarcinoma (β=0.323, p=0.005) were independent prognostic factors for PFS (Table 3).
In the current study, there was a significant positive correlation between PFS and TSR that was calculated only in main mass (R=0.373, p=0.010). There was also a significant positive correlation between PFS and the sum of values those were calculated in all target lesions of RECIST 1.1 criteria (R=0.399, p=0.010) In addition, multivariate analysis also showed that there was a significant positive correlation between TSR and PFS (p=0.029). Presumably, this might be because TSR may be associated with intratumoral heterogeneity of clones that are sensitive to EGFR-TKI. With administration of EGFR-TKI, the size of the tumor will be reduced depending on the fractions of the cells that are responsive to EGFR-TKI7. This leads to the speculation that TSR might be an early indicator for PFS in patients receiving EGFR-TKI therapy.
We have also speculated that there might be a significant correlation between PFS and OS in patients with NSCLC receiving EGFR-TKI therapy. To date, no clinical studies have discussed the PFS as a potential surrogate indicator for OS. Studies, such as IPASS, OPTIMAL, and EURTAC, have not demonstrated that the OS was prolonged. Presumably, this might be due to a cross-over use of EGFR-TKI189. According to the previous BR.21 study performed with patients who had been one or two regimens of combination chemotherapy and not be eligible for further chemotherapy, PFS was a prognostic indicator for OS after a comparison between best supportive care and erlotinib10. There is a correlation between the TSR and PFS. It can therefore be inferred that it might also be used as a predictive indicator for OS.
Most of the patients taking EGFR-TKI experience the disease progression; this indicates that they acquired the resistance. The underlying mechanisms are divided into the resistant mutant development, including T790M point mutation, and clonal selection theory, indicating that there is a decrease in the number of clones that are sensitive to EGFR-TKI but an increase in that of resistant clones 7111213. With regard to the clonal selection theory, there is a growing evidence that patients who previously responded to EGFR-TKI benefit from retreatment with EGFR-TKI. Clonal selection theory is based on intratumoral heterogeneity, thus suggesting that EGFR-TKI-sensitive cells are mixed with EGFR-resistant cells at varying ratios in a single patient or tumor7. With administration of EGFR-TKI, there are changes in the cell population accompanied by the continuous proliferation of EGFR-TKI-resistant cells. These phenomena may lead to tolerance to EGFR-TKI therapy. With the discontinued use of EGFR-TKI, EGFR-TKI-responsive cells can be proliferated. This leads to the speculation that the tumor may be treated with the readministration of EGFR-TKI. One phase II study revealed that gefitinib retreatment can achieve benefits in patients with advanced NSCLC who have controlled previously to gefitinib. In this study, disease control rate was 65.2% that suggest that a second round of gefitinib is worth considering in NSCLC patients with progressive disease after EGFR-TKI failure14.
Even though the cells are drug-sensitive, some of those might remain after administration of EGFR-TKI. It has been known that the drug-sensitive and drug-resistant EGFR-mutant cells exhibited differential growth kinetics, with the drug-resistant cells showing slower growth12. The TSR is an indicator for clones that are sensitive to EGFR-TKI. Therefore, it could be expected that patients who had higher TSR still have relatively higher ratio of sensitive cells after using of EGFR-TKI and will have higher ratio with the lapse of time (Figure 3). Accordingly, TSR can be used to predict the efficacy of the retreatment with EGFR-TKI. We expect that TSR also can be used in evaluating efficiency of next-generation EGFR-TKI on the same principle.
There are some limitations of the current study. First, our study is a non-randomized retrospective one and enrolled a small number of patients in a single-institution. Second, in the current study, patients did not undergo EGFR mutation testing. In a heterogeneous population, we revealed that TSR is significantly correlated with PFS. We are currently conducting a study about the TSR after the first-line of treatment with EGFR-TKI in patients with activating EGFR mutations. Thus, we have shown that the correlation coefficient is significantly higher in patients with activating EGFR mutations as compared with a heterogeneous group of patients (unpublished data). In the current study, we calculated the TSR in a 2-dimensional manner. It would be mandatory to accurately measure the decreased number of tumor cells based on the volume because the mass is a 3-dimensional structure. It is necessary, however, to use the volume using a specially-designed software. Moreover, it would also be difficult to apply the methods of measurement, used in an actual clinical setting.
Our results showed that the TSR might be an early prognostic indicator for PFS in patients receiving EGFR-TKI therapy because it reflexes the ratio of cancer cells harboring sensitive mutation to EGFR-TKI. We also found that there was a significant correlation between the TSR and PFS; this would be of help for predicting the optimal timing of EGFR-TKI treatment. In addition, our results also showed that the TSR might be a prognostic factor for OS because there might be a significant correlation between the PFS and OS.
Finally, it would also be useful in predicting the clinical benefit based on the treatment response and the prognosis in pa tients who are considered to receive re-treatment with EGFR-TKI.
Figures and Tables
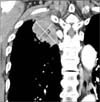 | Figure 1Method of tumor size measurement. The size of the tumor was defined as the section with the longest diameter among the axial, coronal, and sagittal sections of the main mass multiplied by the perpendicular diameter. In this patient, the longest diameter (a) and the longest perpendicular diameter (b) are obtained and multiplied on the coronal section. |
 | Figure 2(A) Correlation of tumor shrinkage rate (TSR) and progression-free survival (PFS). (B) Correlation of TSR calculated in all target lesions of Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria and PFS. (C) Correlation of response rate (%) calculated by sum of diameters according to RECIST 1.1 criteria and PFS. |
 | Figure 3Schema that represents different ratio of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) sensitive cells after administration of EGFR-TKI and discontinuation based on the ratio of EGFR-TKI sensitive cells before treatment. Upper case represents a tumor that has higher ratio of EGFR-TKI sensitive cells than lower case. |
Table 1
Patient characteristics at baseline (n=85)

Table 2
Univariate analyses of PFS

Table 3
Multivariate linear regression analyses of variables for PFS

References
1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957.
2. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-smallcell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388.
3. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128.
4. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–1128.
5. Chen ZY, Zhong WZ, Zhang XC, Su J, Yang XN, Chen ZH, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist. 2012; 17:978–985.
6. Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008; 99:929–935.
7. Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila). 2010; 3:1361–1364.
8. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.
9. Gridelli C, Rossi A. EURTAC first-line phase III randomized study in advanced non-small cell lung cancer: erlotinib works also in European population. J Thorac Dis. 2012; 4:219–220.
10. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005; 353:123–132.
11. Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011; 17:5530–5537.
12. Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011; 3:90ra59.
13. Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009; 10:281–289.
14. Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer. 2012; 77:121–127.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download