Abstract
Lemierre syndrome (LS) is a septic thrombophlebitis of the internal jugular vein (IJV) following an oropharyngeal infection. LS is commonly caused by normal anaerobic flora and treated with appropriate antibiotics and anticoagulation therapy. Although the incidence of disease is very rare, 15% cases of LS are fatal even in the antibiotic era because of disseminated septic thromboemboli. We reported a case of extensive bilateral LS due to methicillin-resistant Staphylococcus epidermidis in a 63-year-old female with lung adenocarcinoma. Initial examination revealed a retropharyngeal abscess; hence, intravenous ceftriaxone and steroid were initiated empirically. However, pulmonary thromboembolism developed and methicillin-resistant S. epidermidis was identified in the bacterial culture. Despite intensive antibiotic and anticoagulation therapies, extensive septic thrombophlebitis involving the bilateral IJV and superior vena cava developed. Adjunctive catheter-directed thrombolysis and superior vena cava stenting were performed and the patient received antibiotic therapy for an additional 4 weeks, resulting in complete recovery.
Lemierre syndrome (LS) is an acute septic thrombophlebitis of the internal jugular vein (IJV) following an oropharyngeal infection1. An annual incidence of LS is 3.6 cases per million people, and LS commonly occurs in healthy young adults and can be complicated by septic emboli to lungs and other organs2. If antibiotics are delayed, LS remains fatal because of disseminated septic thromboemboli2. Although Fusobacterium necrophorum is the most common causative agent, the suggested causative microorganisms include Bacteroides, Eikenella, Streptococcus, Prevotella, methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA)3. Herein, we present a rare case of extensive thrombophlebitis involving bilateral IJV and superior vena cava (SVC) after a retropharyngeal abscess due to methicillin-resistant Staphylococcus epidermidis in a patient with lung adenocarcinoma. In this case, extensive bilateral LS refractory to intensive antibiotic and anticoagulation therapies was successfully managed by adjunctive catheterdirected thrombolysis (CDT) and SVC stenting.
A 63-year-old female presented with dysphagia and facial swelling for 3 days. She had been diagnosed with hypertension and had undergone total thyroidectomy and total hysterectomy due to papillary thyroid carcinoma and endometrial carcinoma, respectively, 10 years prior. She had diagnosed with the adenocarcinoma of the lung (stage IV) 4 months ago, and completed six cycles of cisplatin and premetrexed chemotherapy 2 weeks previously. During the chemotherapy, she exhibited stable disease for adenocarcinoma. At admission (day 1), hyperemia and swelling of the posterior pharyngeal wall were observed. All vital parameters were stable and normal, except for tachycardia (115 bpm). An arterial blood gas showed pH 7.41, PaCO2 19.1 mm Hg, PaO2 137.8 mm Hg, and HCO3 - 11.8 mEq/L, consistent with a chronic compensated respiratory alkalosis with metabolic acidosis along with increased anion gap. White blood cell count (6.3×103/µL), hemoglobin level (13.1 g/µL), platelet count (1.23×103/µL), erythrocyte sedimentation rate (32 mm/hr), and C-reactive protein (1.44 mg/dL) were unremarkable. Routine biochemistry was normal except serum glucose level (426 mg/dL) and glycated hemoglobin level (8.8%). She was newly diagnosed with diabetes, and started on multiple subcutaneous insulin injections.
A computed tomography (CT) scan of the neck revealed a retropharyngeal abscess (Figure 1) and small amount of bilateral pleural effusion. Intravenous ceftriaxone, clindamycin and methylprednisolone (100 mg/12 hr) were initiated empirically after blood cultures. Four days after treatment, dysphagia and facial swelling were not improved and the swelling in both arms and pain to the suprasternal and left supraclavicular regions were additionally developed. Inflammatory changes in mediastinum, pulmonary thromboembolism in the interlobar pulmonary arteries and segmental atelectasis in the right lower lobe were detected using an CT pulmonary angiogram. A repeated laboratory test revealed elevated D-dimer, fibrinogen, and fibrin degradation product levels and methicillinresistant S. epidermidis was identified in the bacterial cultures from the peripheral blood. Anticoagulation therapy was initiated with enoxaparin (60 mg/12 hr) and warfarin (5 mg/day) and antibiotics were changed to intravenous vancomycin, piperacillin/tazobactam and clindamycin for suspected pulmonary thromboembolism and mediastinitis. Follow-up neck CT (day 7) revealed low density materials filling both IJV and SVC.
Despite the intensive medical therapy, the swelling in the face and both arms progressed along with significant leukocytosis with a left shift. On day 8, an enhanced chest CT scan was performed for differential diagnosis, showing extensive occlusive thrombi within the bilateral internal jugular, subclavian and bracheocephalic veins and SVC with lung adenocarcinoma in the left apex and decreased metastatic lesions (Figure 2). CDT, aspiration thrombectomy and SVC endovascular stent placement were performed with 5,000 units of heparin and 500,000 units of urokinase and followed by the overnight infusion of 1,000,000 units of urokinase to relieve the patient's symptoms. Follow-up venography showed residual thrombi in right IJV, thus the repeated aspiration thrombectomy were performed. Final venography revealed the resolution of the thrombosis and intact blood flow in both IJV and SVC (Figure 3A, B). Swelling of the face and both upper arms, and hematology profile were improved 3 days after thrombolysis and the antibiotic and anticoagulation therapies were continued for additional 4 weeks. Recurrence and further complications were not observed over the next 6 months with prolonged anticoagulation therepy (rivaroxaban 20 mg/24 hr) (Figure 3C).
LS is characterized by IJV thrombosis with metastatic infection following a history of pharyngitis affecting peritonsilar tissue. Suggested mechanism includes spread of primary infection into the loose connective tissue and veins2. Although LS is commonly caused by the normal anaerobic flora, a few cases of MRSA-associated LS were reported after 2002. The clinical features of MRSA-associated LS included the unilateral involvement and metastatic spread to the lung4. Most of the cases were successfully treated with tailored antibiotics and anticoagulation therapy. Interestingly, the causative agent in the present case was methicillin-resistant S. epidermidis, which is seldom reported as a cause of LS.
In the present case, occlusive thrombophlebitis involving bilateral IJV, subclavian and bracheocephalic veins and SVC developed and mimicked the SVC syndrome. Several factors might contribute to the vigorous extension of the septic thrombi. First, initial empirical treatment was not appropriate to prevent the development of thrombophlebitis caused by methicillin-resistant S. epidermidis. Once septic thrombi develop, response to appropriate antibiotics in thrombophlebitis is usually slow due to poor antibiotic penetration into the fibrin clot5. Furthermore, the S. epidermidis adhering to the endothelium have a significantly higher antimicrobial tolerance than do planktonic cells, which could result in insufficient efficacy of the antibiotics tailored according to the culture and sensitivity results6. Second, we used a systemic steroid to secure the airway. Corticosteroid administration was suggested to promote the spread of throat infections by compromising in vivo humoral immune defense mechanisms7. The vigorous extension of bilateral thrombophlebitis might be attributed to the high-dose systemic steroids used in our case. Third, coexisting lung adenocarcinoma might play a role in massive thrombi formation. Lung adenocarcinoma is a leading cancer causing hypercoagulability related to malignancy. A large cohort study showed that the incidence of venous thrombosis is >20-fold increased in the patients with lung cancer, and the risk is higher in the patients with adenocarcinoma than in the patients with squamous cell cacrcinoma8. The pathophysiological mechanisms include production of mucin which results in platelet-rich microthrombi, precoagulative factors, including tumor necrosis factor-1, interleukin (IL)-1, IL-6, factor XII and overexpression of tissue factors, resulting in activation of the extrinsic clotting pathway910. We think that a compromised immune system after chemotherapy might contribute to the development of LS in this case, and the co-existing lung adenocarcinoma affected the vigorous extension of the septic thrombi.
Prolonged antibiotic treatment is necessary for the complete resolution of LS. A 6-week antibiotic course is typically considered sufficient for adequate penetration into the fibrin clot and to prevent relapse3. Massive thrombosis involving bilateral IJV and retrograde spread of thrombus are considered indications for anticoagulation therapy11. Although the use of low-molecular-weight heparin has been reported beneficial in terms of early clinical improvement, our patient underwent a rapid progression of thrombophlebitis and worsening of the symptoms despite the intensive antibiotic and anticoagulation therapies12. Because the patient underwent hemodynamic instability due to extensive thrombophlebitis involving bilateral IJV and SVC, we performed CDT and SVC stenting to recover promptly the venous flow and to enhance the antibiotic penetration in spite of possible dissemination of septic thromboemboli. The patient had a favorable outcome without development of further complications.
In conclusion, LS can be accompanied by massive thrombi extending to SVC, and masquerade as SVC syndrome in a patient with lung adenocarcinoma. Therefore, physicians should be aware of the LS as a differential diagnosis in a lung cancer patient with cryptogenic facial swelling and dyspnea, and the methicillin-resistant S. epidermidis as a causative agent of LS. Within the limitations of this case report, we believe that adjunct use of CDT and SVC stenting can be considered in massive thrombophlebitis refractory to appropriate antibiotic and anticoagulation therapies.
Figures and Tables
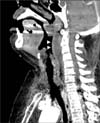 | Figure 1Sagittal images from an initial contrast-enhanced neck computed tomography scan show a retropharyngeal abscess (arrowheads). |
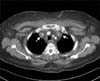 | Figure 2Axial computed tomography images of the thorax show enlarged bilateral internal jugular veins and thrombosis (arrows). |
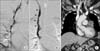 | Figure 3(A) Initial venography shows impaired blood flow in the right internal jugular vein (IJV) and superior vena cava (SVC). (B) Follow-up venography reveals improved blood flow after catheter-directed thrombolysis and SVC stenting (arrowheads). (C) Computed tomography at 6 months after treatment shows resolution of the thrombosis and intact blood flow in both IJV and SVC (arrows). |
References
1. Lamierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936; 227:701–703.
2. Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre's syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012; 12:808–815.
3. Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre's syndrome. Clin Microbiol Rev. 2007; 20:622–659.
4. Chanin JM, Marcos LA, Thompson BM, Yusen RD, Dunne WM Jr, Warren DK, et al. Methicillin-resistant Staphylococcus aureus USA300 clone as a cause of Lemierre's syndrome. J Clin Microbiol. 2011; 49:2063–2066.
5. Riordan T, Wilson M. Lemierre's syndrome: more than a historical curiosa. Postgrad Med J. 2004; 80:328–334.
6. Costa AR, Henriques M, Oliveira R, Azeredo J. The role of polysaccharide intercellular adhesin (PIA) in Staphylococcus epidermidis adhesion to host tissues and subsequent antibiotic tolerance. Eur J Clin Microbiol Infect Dis. 2009; 28:623–629.
7. Righini CA, Karkas A, Tourniaire R, N'Gouan JM, Schmerber S, Reyt E, et al. Lemierre syndrome: study of 11 cases and literature review. Head Neck. 2014; 36:1044–1051.
8. Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost. 2004; 2:1760–1765.
9. Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003; 349:109–111.
10. Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007; 110:1723–1729.
11. McGouran D, Keene A, Walklin R, Carter J. A complex case of bilateral Lemierre syndrome with suggestions on anticoagulation management. Intern Med J. 2013; 43:728–730.
12. Bach MC, Roediger JH, Rinder HM. Septic anaerobic jugular phlebitis with pulmonary embolism: problems in management. Rev Infect Dis. 1988; 10:424–427.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download