Abstract
Cryptococcal pneumonia usually occurs in immunocompromised patients with malignancy, acquired immune deficiency syndrome, organ transplantations, immunosuppressive chemotherapies, catheter insertion, or dialysis. It can be diagnosed by gaining tissues in lung parenchyma or detecting antigen in blood or bronchoalveolar lavage fluid. Here we report an immunocompetent 32-year-old male patient with diabetes mellitus diagnosed with cryptococcal pneumonia after a ultrasound-guided percutaneous supraclavicular lymph node core needle biopsy. We treated him with fluconazole at 400 mg/day for 9 months according to the guideline. This is the first case that cryptococcal pneumonia was diagnosed from a percutaneous lymph node biopsy in South Korea.
Cryptococcosis is a fungal infection that can result in pulmonary involvement after inhalation of Cryptococcus neoformans spores1. This fungus is ubiquitous, being commonly found in aged bird droppings but also in soil and decayed wood2. Pulmonary cryptococcosis occurs not only in immunocompromized patients such as those with acquired immune deficiency syndrome, hematological malignancies and corticosteroid therapies, but also in healthy individuals3. Pulmonary infections are rare in individuals with normal immunity4. In immunocompetent hosts, cryptococcosis is more likely to be limited to the lung and occurs more commonly in patients with chronic lung disease5.
Cryptococcal pneumonia is usually diagnosed by gaining tissues of lung parenchyma through surgery or computed tomography (CT)-guided transthoracic fine needle aspiration biopsy and detecting antigen in blood or bronchoalveolar lavage fluid (BALF). Also it sometimes accompanies by meningitis. So we can diagnose it by detecting cryptococcal antigen in cerebrospinal fluid (CSF). Until now, most cases of cryptococcal pneumonia had been diagnosed mainly by obtaining tissues of lung parenchyma from a CT-guided percutaneous fine needle aspiration biopsy in South Korea. We report the first case of cryptococcal pneumonia diagnosed by a percutaneous supraclavicular lymph node biopsy in South Korea. In addition, we had treated him with fluconazole successfully, achieving clinical and radiological improvement.
A 32-year-old man was referred to our division of pulmonology from a clinic for further evaluation and management of pneumonia. He was a never smoker and had no history of pulmonary tuberculosis or allergy, but had been taking medications for diabetes mellitus for 3 years. He presented with complaints of fever, cough, sputum, and pleuritic pain which had appeared 1 month before he visited to our institution. He did not complain of headache, nausea or vomiting and show Brudzinski neck sign, Kernig sign or stiff neck. Ten days ago, he visited to the clinic and was diagnosed with bacterial pneumonia. Therefore, he had received antibiotic for 8 days with admission. However, there was no improvement in his condition.
When he visited to our hospital for the first time, he presented with a blood pressure of 138/81 mm Hg, a heart rate of 96 per minute, a respiratory rate of 20 per minute, and a body temperature of 36.6℃.
Physical examination of the chest revealed clear breathing sound without wheezing or crackle. Lymph node was not palpated in the both neck area.
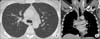
Chest radiograph and CT scans of the chest revealed innumerable small lung nodules, interlobular septal thickening, airspace consolidation in both lungs with pleural effusion and enlarged lymph nodes in supraclavicular, mediastinal, hilar, interlobar, subcarinal, and paraesophageal areas bilaterally (Figure 1).
The findings of laboratory tests showed a white blood cell count of 12,800/µL (neutrophil 47%, lymphocyte 25%, and eosinophil 18.9%), a C-reactive protein of 9.71 mg/dL, and a erythrocyte sedimentation rate of 81 mm/hr. We could not gain any positive result from blood cultures, sputum stain and cultures and acid-fast bacilli stain and cultures. We also could not find out any parasite antibody, bacterial DNA or cryptococcal antigen in the peripheral blood.
We assessed his blood sugar control state. The findings of laboratory tests showed a HbA1c of 10.9%, a C-peptide of 1.95 ng/mL, and a insulin of 9.28 µU/mL.
We checked the immune status of him. Antigen and antibody of human immunodeficiency virus (HIV) were not detected. The findings of laboratory tests related with immunity showed a C3 of 188 mg/dL (normal range, 90-180 mg/dL), a C4 of 46 mg/dL (normal range, 10-40 mg/dL), a CH50 of 67.34 mg/dL (normal range, 41.25-94.3 mg/dL), a CD4 (T-helper cell) of 33.89, a CD8 (T-suppressor cell) of 27.71, and a CD4/CD8 of 1.25 (normal range, 1-4). The serum level of IgG was 1,321 mg/dL (normal range, 700-1,600 mg/dL). The serum level was IgA was 114 mg/dL (normal range, 70-400 mg/dL). The serum level of IgM was 206 mg/dL (normal range, 40-230 mg/dL). The serum level of IgE was 4,051 IU/mL (normal range, 0.1-200 IU/mL).
Inspite of treatment with intravenous cefoperazone/sulbactam and oral azithromycin, symptoms and the findings of the chest radiograph did not improve. Therefore, we decided to perform bronchoscopy and bronchoalveolar lavage. On the bronchoscopy, we found severe inflammation in left bronchial tree. An analysis of BALF revealed a white blood cell count of 860/µL (neutrophil 13%, lymphocyte 80%, and monocyte 7%), negative findings on bacterial stain and culture, acid-fast bacilli stain and culture, viral cultures (parainfluenza, adenovirus, and respiratory synocytial virus), fungal culture and pneumocystis jirovecy polymerase chain reaction (PCR). We identified some microorganisms which were looking like protozoas.
As results were inconclusive, we performed ultrasound-guided percutaneous left supraclavicular lymph node core needle biopsy. We stained the tissues with mucicarmine, periodic acid-Schiff, Grocott-Gomori's methenamine silver (Figure 2). These histopathological data led to the conclusion that he had been suffering from cryptococcal pneumonia. We took a lumbar puncture to exclude cryptococcal meningitis. There was no evidence of meningitis. The lab findings of CSF tapping showed a white blood cell count of 1/µL, a protein of 18.0 mg/dL, and a glucose of 91 mg/dL. We did not detect any microorganism on CSF tests including cryptococcal antigen test, India ink test, fungus cultures, Gram stain and culture, viral PCR (herpes simplex virus and varicella zoster virus), and tuberculosis PCR.
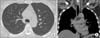
He was treated with fluconazole, 400 mg/day orally for 9 months according to the guideline6. He got a marked improvement in his symptoms such as fever, cough, and sputum. At his 3- and 9-month follow-ups, chest radiographs and CT scans of the chest showed a significant reduction of the innumerable small nodules and lymphadenopathy bilaterally. (Figures 3, 4)
Cryptococcus neoformans is an encapsulated, round to oval yeast that is surrounded by a polysaccharide capsule. There are two pathogenic variants: C. neoformans var. neoformans and C. neoformans var. gattii7. Although the mechanism for cryptococcal pathogenesis has been poorly understood3, C. neoformans usually affects people with HIV, but C. gattii primarily causes disease in immunocompetent patients8. Cryptococcal infections occur predominantly in patients with T-cell mediated immune defects2. The majority of cases of cryptococcal disease occur in persons with a CD4 cell count below 200 cells/µL, usually below 50 cells/µL7. Thomas et al.9 reported that 30 of the HIV-negative 69 patients had diabetes mellitus and among the HIV-positive cases, 2% had had DM. In this study, the frequency of DM in the HIV-negative cryptococcosis population is four times higher than the U.S. population estimates for DM. Furthermore, of all HIV-negative diabetic cryptococcosis cases, nine had DM without another immunocompromising condition, suggesting a 2.6 higher risk9. Nadrous et al.2 reported that 6 of 143 patients (excluding patients who were HIV positive and were organ transplant recipients) from over the 26-year period survey (1976-2001)2. The immunity of the patient in this case was intact without HIV or malignancy. And the findings of lab tests related with immunity were within the normal range.
The respiratory tract is thought to be the entry site. Depending on a patient's immune status, spores may remain dormant in the lung or may undergo hematogenous spread to any organ system1. Cryptococci have a tropism for the central nervous system (CNS); after the CNS, the respiratory system is the most commonly affected. It is known that 10% of pulmonary cryptococcosis cases are complicated with cryptococcal meningoencephalitis regardless of the existence of underlying diseases. However, infection has been reported in virtually every organ in body3.
Cryptococcal infection limited to the lung is defined as primary pulmonary cryptococcosis, which is difficult to diagnose because symptoms and radiologic findings are non-specific. Serologic tests have an accuracy of 87% in patients with disseminated cryptococcosis, but only 30% in patients with primary pulmonary cryptococcosis4.
The presentation of pulmonary cryptococcosis can range from asymptomatic nodular disease to severe acute respiratory distress syndrome (ARDS)10. Immunocompromised patients are much more likely to present with fulminant infection that can progress to ARDS5. Up to 10% of patients with disseminated cryptococcosis may also present with acute respiratory failure7. Immunocompetent subjects with pulmonary cryptococcosis usually are asymptomatic or have mild symptoms that include fever, cough, sputum, myalgia, and dyspnea5. Sometimes pleural symptoms may predominate5. The patient in this case had complained of fever, cough and sputum for 1 month before he came to our hospital.
Physical examination of the chest may reveal inspiratory crackles, evidence of consolidation or pleural effusion7. The chest examination of the patient in the case showed clear breath sounds without rale or wheezing.
The eosinophils accounted for 18.9% of white blood cells. We believed that eosinophilia was caused by the antibiotic prescribed before he came to our hospital.
The diagnosis of pulmonary cryptococcosis is usually established by smear or culture of sputum, BALF, bronchial washing and occasionally pleural fluid7 in a patient who has clinical symptoms and radiographic findings compatible with cryptococcosis5. In a patient who has no radiographic findings related with Cryptococcus, isolating C. neoformans from sputum may reflect colonization. Cryptococcus species grow readily on standard agar media and can be easily detected by clinical laboratories. However, we couldn't obtain any meaningful results from the tests mentioned above.
A cryptococcal antigen test is highly sensitive and specific. So it is routinely used to establish the diagnosis of cryptococcal meningitis and disseminated cryptococcosis. However, the serum cryptococcal antigen test is often negative in patients who have isolated pulmonary. Anyway, cryptococcal pneumonia sometimes accompanies by meningitis, cryptococcal antigen can be also detected in CSF. The presence of a positive serum cryptococcal antigen titer implies deep tissue invasion and a high likelihood of disseminated disease10. In fact, a positive serum cryptococcal antigen test in a solid organ transplant recipient who has pulmonary cryptococcosis has been shown to reflect extrapulmonary or more severe pulmonary disease5. We could not detect any cryptococcal antigen from blood, BALF, and CSF.
Since pulmonary cryptococcosis occurring in immunocompetent hosts is a relatively rare manifestation, typical radiographic findings are not firmly established yet. However, several previous studies stated similar findings that can be considered as relatively established findings of pulmonary cryptococcosis in immunocompetent hosts. The most common radiographic feature was well or ill defined pulmonary nodules or masses. Other findings included areas of segmental or lobar consolidations, ground glass opacities, and miliary disease. Cavitation, pleural effusions, and lymphadenopathies were also recognized features. Generally, there were tendencies of immunocompromised hosts showing higher incidence of pulmonary parenchymal abnormalities, on the contrary, immunocompetent hosts showed higher incidence of pulmonary nodules and masses111213. Initial CT scan of the chest in this case showed innumerable small lung nodules, multiple enlarged lymph nodes and pleural effusion.
Histopathology of biopsy specimens from CT-guided transthoracic fine needle aspiration biopsy, transbronchial biopsy, surgical lung biopsy, and pleural biopsy can also be diagnostic. As new cutaneous lesions may be signs of dissemination, their sudden appearance should be promptly considered as an indication for skin biopsy7. Mucicarmine, a specific stain for cryptococcus, should always be performed to identify yeast-like structures in tissues. In tissues, a clear zone, representing the capsule around the yeast, is sometimes seen. We do not find out even a case of cryptococcal pneumonia which was diagnosed by a percutaneous lymph node biopsy in South Korea. We confirmed cryptococcosis by a percutaneous supraclavicular lymph node biopsy and mucicarmine stain.
In immunocompetent patients with pulmonary cryptococcosis, consider a lumbar puncture to rule out asymptomatic CNS involvement. However, for normal hosts with asymptomatic pulmonary nodule or infiltrate, no CNS symptoms and negative or very low serum cryptococcal antigen, a lumbar puncture can be avoided (B-II)6. Because there are so many disseminated infections in immunocompromised patients, a systematic evaluation, including cultures from blood and CSF and measurement of serum and CSF cryptococcal antigen, should be performed in all immunosuppressed patients who present with seemingly isolated pulmonary cryptococcosis5. According to the guideline6, we took a lumbar puncture to decide treatment regimen. We couldn't find out any evidence of cryptococcal meningitis.
Pulmonary crytococcosis in a patient with no identifiable immunologic defects may resolve spontaneously and not require antifungal therapy, althoughit can occasionally be severe and deadly2. In immunocompromised patients, cryptococcosis can be severe and rapidly progressive, often accompanied by disseminated infection, including the CNS disease7, so it requires prolonged systemic antifungal therapy2. The goal of treatment is cure of the cryptococcal infection and prevention of dissemination of disease to the CNS10. Fluconazole is active against C. neoformans, is easily administered, and has an excellent safety profile14. Immunocompetent patients who are asymptomatic and who have a culture that is positive for C. neoformans may be observed carefully or treated with fluconazole, 200-400 mg/day for 3-6 months (AIII)10. Immunocompetent patients who present with mild-to-moderate symptoms should be treated with fluconazole, 400 mg/day orally for 6-12 months; persistently positive serum cryptococcal antigen titers are not criteria for continuance of therapy (B-II). For severe disease, treat similarly to CNS disease (B-III)10. Itraconazole (200 mg twice per day orally), voriconazole (200 mg twice per day orally), and posaconazole (400 mg twice per day orally) are acceptable alternatives if fluconazole is unavailable or contraindicated (B-II)6. The toxicity of amphotericin B limits its utility as a desired agent in the treatment of mild-to-moderate pulmonary disease among immunocompetent hosts. However, if oral azole therapy cannot be given, or the pulmonary disease is severe or progressive, amphotericin B is recommended, 0.4-0.7 mg/kg/day for a total dose of 1,000-2,000 mg10. Early, appropriate treatment of non-CNS pulmonary and extrapulmonary cryptococcosis reduces morbidity and prevents progression to potentially life-threatening CNS disease10. As our patient was immunocompetent and had moderate symptoms, we chose to prescribe fluconazole, 400 mg/day orally for 9 months to him according to the guideline6. After 9 months treatment, all of his symptoms went away and cryptococcosis related lesions in his lungs disappeared in his follow-up image tests.
Figures and Tables
Figure 1
Initial lung images. (A) Initial chest radiograph showing diffuse reticulonodular opacities in both lungs. (B) Initial chest computed tomography (CT) scan shows innumerable small nodules, interstitial thickening, and ground glass attenuation in both lungs. Note small bilateral pleural effusions. Both hilar regions are enlarged, representing lymphadenopathy. (C) Chest CT mediastinal window setting coronal image showing enlarged lymph nodes in both supraclavicular areas, both mediastinal, interlobar, and subcarinal areas.

Figure 2
Supraclavicular lymph node biopsy. (A) Granulomatous inflammation in cryptococcosis showing numerous multinucleated giant cells (H&E stain). (B) The periodic acid-Schiff stain reveals yeast forms of Cryptococcus separated from the cell cytoplasm by a clear space. (C) The capsule of Cryptococcus stained bright-red with mucicarmine stain (A, ×100; B and C, ×400).

References
1. Chang WC, Tzao C, Hsu HH, Lee SC, Huang KL, Tung HJ, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest. 2006; 129:333–340.
2. Nadrous HF, Antonios VS, Terrell CL, Ryu JH. Pulmonary cryptococcosis in nonimmunocompromised patients. Chest. 2003; 124:2143–2147.
3. Nakamura S, Miyazaki Y, Higashiyama Y, Yanagihara K, Ohno H, Hirakata Y, et al. Community acquired pneumonia (CAP) caused by Cryptococcus neoformans in a healthy individual. Scand J Infect Dis. 2005; 37:932–935.
4. Chang YS, Chou KC, Wang PC, Yang HB, Chen CH. Primary pulmonary cryptococcosis presenting as endobronchial tumor with left upper lobe collapse. J Chin Med Assoc. 2005; 68:33–36.
5. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012; 17:913–926.
6. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010; 50:291–322.
7. Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology. 2009; 14:474–485.
8. Johannson KA, Huston SM, Mody CH, Davidson W. Cryptococcus gattii pneumonia. CMAJ. 2012; 184:1387–1390.
9. Thomas CJ, Lee JY, Conn LA, Bradley ME, Gillespie RW, Dill SR, et al. Surveillance of cryptococcosis in Alabama, 1992-1994. Ann Epidemiol. 1998; 8:212–216.
10. Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000; 30:710–718.
11. Fox DL, Muller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol. 2005; 185:622–626.
12. Lindell RM, Hartman TE, Nadrous HF, Ryu JH. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology. 2005; 236:326–331.
13. Hung MS, Tsai YH, Lee CH, Yang CT. Pulmonary cryptococcosis: clinical, radiographical and serological markers of dissemination. Respirology. 2008; 13:247–251.
14. Guy JP, Raza S, Bondi E, Rosen Y, Kim DS, Berger BJ. Cryptococcus pneumonia presenting in an immunocompetent host with pulmonary asbestosis: a case report. J Med Case Rep. 2012; 6:170.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download