Abstract
Eosinophilic lung diseases are heterogeneous disorders characterized by varying degrees of pulmonary parenchyma or blood eosinophilia. Causes of eosinophilic lung diseases range from drug ingestion to parasitic or fungal infection as well as idiopathic. The exact pathogenesis of eosinophilic lung disease remains unknown. Urushiol chicken can frequently cause allergic reactions. Contact dermatitis (both local and systemic) represents the most-common side effect of urushiol chicken ingestion. However, there has been no previous report of lung involvement following urushiol chicken ingestion until now. A 66-year-old male was admitted to our hospital with exertional dyspnea. Serial chest X-ray revealed multiple migrating infiltrations in both lung fields, with eosinophilic infiltration revealed by lung biopsy. The patient had ingested urushiol chicken on two occasions within the 2 weeks immediately prior to disease onset. His symptoms and migrating lung lesions were resolved following administration of oral corticosteroids.
Contact dermatitis is a typical side-effect of lacquer ingestion, which has a documented history dating back to 453 BC. Lacquer is rarely ingested outside of Korea. The side-effects of lacquer-induced, or systemic, contact dermatitis include not only skin lesions, but also fever (20% of cases), chills (15%), and digestive symptoms (13%) such as abdominal pain, nausea, vomiting, and diarrhea. In severe cases, breathing difficulties or shock may develop1.
Eosinophilic lung disease is characterized by excessive eosinocytes in lung lesions, which are believed to play important pathological and physiological roles. According to the eosinophilic lung disease classification system suggested by Allen and Davis2, symptoms can include increased eosinocytes in peripheral blood, lung infiltration in chest X-ray images, eosinocyte infiltration in lung biopsy, and an increase in the eosinocyte ratio in bronchoalveolar lavage fluid. Eosinophilic lung can be diagnosed even without a significant increase in eosinocytes in the peripheral blood during early or recovery stages23.
We herein report a case of eosinophilic lung infiltration following Rhus chicken ingestion.
A 66-year-old male visited our hospital due to difficulty breathing when walking uphill for 7 days. The patient ingested Rhus chicken on two occasions over a 2-week period immediately prior to admission to our hospital. Three days after his second Rhus chicken ingestion, the patient experienced chills and sputum production. During the week prior to visiting our hospital as an outpatient, he experienced breathing difficulties when walking quickly or uphill. The patient had been treated for hypertension for the past 10 years, and was taking warfarin for the previous 3 months due to cerebral infarction and atrial fibrillation. There was no history of allergies. He has no specific family history.
On admission, he was acutely ill-looking. Blood pressure was at 150/80 mm Hg, pulse rate was 78 beats per minute, and body temperature was 37.9℃. No tenderness was experienced in the paranasal sinus area, and there were no specific findings from anterior rhinoscopy and oropharyngeal examinations. No cervical lymph node was palpated, and there was no carotid artery dilation. In the patient's chest auscultation, rale was confirmed in both lower lobes. No pretibial pitting edema was observed. Peripheral blood tests revealed a white blood count of 7,080/µL (neutrophils, 56.1%; eosinocytes, 1.6%; lymphocytes, 35.7%; monocytes, 6.2%; and basophils, 0.4%), hemoglobin at 11.7 g/dL (reference value, 12-16 g/dL), a platelet count of 182,000/µL (reference value, 30,000-450,000/µL), C-reactive protein at 1.77 mg/dL (reference value, 0.01-0.29), and serum total IgE at 432 kU/L (reference value, 0-100 kU/L). The results of liver and kidney function, and urine tests, were normal. Blood, urine, and sputum bacterial culture tests were negative. Parasite tests, including the lung fluke test, were negative. The international normalized ratio was 1.86 (reference value, 0.8-1.2). In the electrocardiogram, mild premature ventricular contractions were observed. Cardiac echography revealed that the ejection fraction was well-maintained at 59% with normal function. In the lung function test, forced vital capacity (FVC) was at 2.3 L (64% predicted) with forced expiratory volume in 1 second (FEV1) at 1.93 L (76% predicted) and the FEV1/FVC ratio at 84% predicted, indicating restrictive ventilatory impairment. The diffusing capacity of carbon monoxide (DLCO) was 32% predicted. In the arterial blood test, the PaO2 was 60, confirming hypoxia. In bronchoalveolar lavage fluid, macrophagocytes were at 54%, neutrophils at 39.4%, eosinocytes at 5.2%, and lymphocytes at 3%.
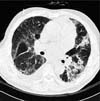
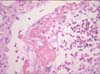
In plain chest X-ray images obtained on the day of admission, lung infiltration was observed in the left-upper lobe. Infiltration disappeared after 5 days, but re-emerged in the right-upper lobe. After 11 days right-upper lobe infiltration improved, but left-upper lobe infiltration increased in extent (Figure 1). In high-definition chest computed tomography images, right and left-upper and lower lobes were observed with multiple lung infiltration and glass opacity (Figure 2). In bronchoscopic images, also obtained on day 11, bleeding in the peribronchial area of the left-upper lobe and increased sputum production were observed. Accordingly, a bronchoalveolar lavage fluid test was conducted, confirming a significant increase in eosinocytes. Following percutaneous transthoracic needle biopsy of the left-upper lobe lesion, eosinocyte infiltration was confirmed (Figure 3).
To treat fever and the increasing breathing difficulties caused by migratory lung infiltration, 40 mg of prednisolone were administered orally. Clinical symptoms, including fever and breathing difficulties, improved. Lesions in plain chest X-ray images also improved rapidly. Plain chest X-ray images obtained after 4 weeks revealed that lung infiltration had completely disappeared.
The sap of the defoliating lacquer tree (Rhus verniciflua, Anacardiaceae) has been used to coat traditional furniture and bowls, and as a hair dye. In traditional Korean medicine, lacquer has been used as a digestive system medicine or as vermifuge. Worldwide, there are approximately 70 lacquer tree families and 600 species. In Korea, one family and six species have been located1, but have not yet been subjected to cross-antigenicity and antigen standardization.
In Korea, Rhus chicken is mistakenly believed to confer benefits for menstrual irregularity and pain, muscle pain, and gastrointestinal tract disorders, and has been traditionally used as a health supplement. The most common lacquer-related disease is contact dermatitis, which develops following contact with the tree. Korea is the only country in which contact dermatitis has been reported subsequent to the ingestion of lacquer tree sap. No such cases have been reported in China or Japan. According to the Korea Food and Drug Administration (KFDA) raw materials classification system, the lacquer tree belongs to the "frequently ingested inedible animal/plant materials," group, and to a subcategory of "raw materials with strong or toxic pharmaceutical functions." Since 2005, however, lacquer tree sap has been used as an ingredient in Rhus chicken and duck dishes, following removal of the sap's toxic ingredient, urushiol1.
Cases of clinical side effects following Rhus chicken ingestion are common, of which the most-frequently reported is systemic contact dermatitis. In severe cases, acute systemic exanthematous pustulosis may develop4, but no case of lung infiltration has yet been reported. The majority of patients visiting health care providers complain of skin pathologies such as mild rash, wheal, or itchiness. Symptoms are typically mild, such that symptomatic treatments are sufficiently effective, which can lead to under-reporting.
Urushiol, a liquid from the lacquer tree distilled at between 210℃-222℃, is a mixture of four catechol derivatives, pentadecylcatechols. The sap resin oleoresin contains urushiol, which in turn contains the strong antigenic material pentadecylcathecol, which is associated with various allergic reactions. When the pentadecylcatechol side-chain is unsaturated, antigenicity increases, and decreases when it is saturated or replaced with a catechol ring5. These substances may precipitate allergic reactions, but the underlying mechanism of eosinocyte stimulation and allergy development is not yet fully understood.
According to the eosinophilic pneumonia mechanism described by Kanner and Hammar6, the immune complex formed by pentadecylcatechol produces factors that shift the eosinophilic chemotactic factor or eosinocytes into the parenchyma. Eosinocytes released in the parenchyma can destroy lung proteins. Type 1 and 3 hyper-reactions may be involved in the development of chronic eosinophilic pneumonia67.
Our patient complained of breathing difficulties without skin symptoms 4 days post-sap ingestion. Upon admission, he exhibited mild fever and non-purulent sputum. He was initially administered intravenous antibiotic injections due to community-acquired pneumonia, but migratory lung infiltration was observed in his chest X-ray such that Loeffler's syndrome was suspected. Detailed medical history confirmed that he had eaten Rhus chicken twice immediately prior to admission. In bronchoscopy, a slight increase in eosinocytes was observed in the bronchoalveolar lavage fluid, at the peribronchial area of the left-upper lobe, where sputum had also increased. This was not a tumor lesion, but a percutaneous transthoracic needle biopsy was performed in the left-upper lobe where infiltration had increased. Eosinocyte infiltration was confirmed and steroids were administered, following which the patient rapidly recovered. For ethical reasons, clinical experiments concerned with Rhus chicken ingestion have not been conducted.
Eosinophilic lung disease is diagnosed when the level of peripheral blood eosinocytes increases and lung infiltration is observed in chest X-ray images, and when eosinocyte infiltration is observed in lung biopsy regardless of the extent of increase in the peripheral blood eosinocytes and when the eosinocyte ratio in the bronchoalveolar lavage fluid increases. The percentage of eosinophila is supposed to be more than 25% in bronchoalveolar lavage (BAL) fluid in case of acute eosinophilic pneumonia. Normal BAL fluid consists of less than 1% eosinophils8. It has been reported the correlations between the size of infiltrations in radiologic findings and inflammation in BAL fluid in the various kinds of interstitial pneumonia, although there is few report about only for eosinophilic pneumonia910.
In lung biopsy, mild eosinocyte infiltration was observed in the parenchyma, and numerous eosinocytes were observed in the pulmonary alveoli. Accordingly, eosinocyte-associated immune reactions were assumed to have occurred. An eosinophilic lung can be diagnosed in the absence of a significant increase in eosinocytes during early or recovery stages3. The patient might have already been in recovery when the biopsy was conducted, or tissues might have been insufficient due to percutaneous transthoracic needle biopsy. The amount of eosinophil in bronchoalveolar lavage was too low for a diagnosis of acute eosinophilic pneumonia, possibly due to a low degree of infiltration and migration to another site. It is also possible that, because the patient was in early-phase hypersensitivity pneumonitis, neutrophils represented the major inflammatory cells involved in the pathogenesis1112. In several lung diseases, eosinocyte level can be a useful diagnostic index to determine whether treatment with corticosteroids should be applied2.
The patient rapidly recovered following oral intake of steroids. Outpatient chest radiography during the follow-up revealed that diffuse lung infiltration was completely attenuated.
Several skin-related side effects of Rhus chicken ingestion have been reported, but we herein document for the first time a case of eosinophilic lung infiltration following Rhus chicken ingestion.
Figures and Tables
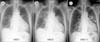 | Figure 1(A-C) Chest X-ray revealing decreased peribronchial opacity in the left-upper lobe (LUL) and new infiltrations in the right lung after 5 days of urushiol chicken ingestion. On day 11, infiltration in the LUL increased again. |
References
1. Park SD. Herb medicine-induced adverse effects in dermatological field. J Korean Med Assoc. 2005; 48:325–332.
2. Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994; 150(5 Pt 1):1423–1438.
3. Choi DC. Eosinophilic lung diseases: diagnosis and treatment. Korean J Med. 2009; 76:274–281.
4. Shim IK, Choi YJ, Lee SJ, Lee HW, Choi GS, Kim HK, Suh KS. A case of acute generalized exanthematous pustulosis with patterns of Septic Shock. Korean J Med. 2011; 81:802–806.
5. Won TH, Seo PS, Park SD, Kim DL, Park JH. Clinical features in 147 patients with systemic contact dermatitisdue to the ingestion of chicken boiled with Japanease lacquer tree. Korean J Dermatol. 2008; 46:761–768.
6. Kanner RE, Hammar SP. Chronic eosinophilic pneumonia. Ultrastructural evidence of marked immunoglobulin production plus macrophagic ingestion of eosinophils and eosinophilic lysosomes leading to intracytoplasmic Charcot-Leyden crystals. Chest. 1977; 71:95–98.
7. Yoon JH, Lee JW, Kim JE, Kim DH, Park SH, Kim WJ, et al. Two cases of acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Korean J Asthma Allergy Clin Immunol. 2012; 32:131–135.
8. Jeong YJ, Kim KI, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007; 27:617–637.
9. Veeraraghavan S, Latsi PI, Wells AU, Pantelidis P, Nicholson AG, Colby TV, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J. 2003; 22:239–244.
10. Biederer J, Schnabel A, Muhle C, Gross WL, Heller M, Reuter M. Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol. 2004; 14:272–280.
11. Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012; 185:1004–1014.
12. Wells AU. The clinical utility of bronchoalveolar lavage in diffuse parenchymal lung disease. Eur Respir Rev. 2010; 19:237–241.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download