Abstract
Metastatic squamous cell carcinoma from a cancer of unknown primary (CUP) affecting the intrathoracic lymph node is very rare. We reported a case of metastatic squamous cell carcinoma in the hilar and interlobar lymph node from a patient with CUP and reviewed the associated literature. Abnormal mass in the right hilar area was incidentally detected. A chest computed tomography scan showed a 2.5-cm diameter mass in the right hilum that had changed little in size for 3 years. The patient underwent a right pneumonectomy and mediastinal lymph node dissection. A metastatic squamous cell carcinoma in the hilar and interlobar lymph nodes without a primary lung or other lesion was diagnosed. The patient received adjuvant chemotherapy for a diagnosis of T0N1M0 lung cancer.
Cancers of unknown primary (CUP) occur approximately in 3% to 5% of all cancer1,2. General characteristics of CUPs consist of early dissemination, unpredictable metastatic pattern and aggressiveness2,3. More than 50% of patients with CUP have metastasis affecting multiple sites and the rest present with only a single site of involvement4. Lymph node is one of the most common site of metastasis, followed by the liver, lung, and bone5. But metastases into mediastinal or hilar lymph nodes in patients with CUP are rare and have been reported in a few literatures6,7,8,9,10,11,12. Since only 5% of patients with CUP are squamous cell carcinomas13, a metastatic squamous cell carcinoma of mediastinal or hilar lymph node from a CUP is even rarer and have been seldomly reported6,7,8,10,11,12. We report a quite rare case of metastatic squamous cell carcinoma from a CUP in the hilar and interlobar lymph node that had a very indolent clinical course during 3 years. A review of the associated literatures is also presented.
A 59-year-old Korean man had a medical examination for healthy check-up in December of 2012. An abnormal mass-like lesion in the right hilum was incidentally detected on chest X-ray without any symptom. He was referred to our center for further evaluation.
The patient has hypertension, diabetes mellitus and a history of angina. He visited the division of cardiology of our hospital in March of 2010, and was diagnosed with angina and hypertension. A cardiac angio computed tomography (CT) scan performed at that time detected a 2.5-cm diameter, minimally enhanced mass in the right hilar area (Figure 1A). Since then, he failed to return and was lost to follow up. The patient had a 40 pack-year cigarette smoking history and a family history of stomach cancer. On this admission, the physical examination was unremarkable. The blood examination showed eosinophilia (eosinophil 18.5%, 1,750/µL) and the rest of the laboratory tests including serum tumor markers (carcinoembryonic antigen, cancer antigen 19-9, alpha-fetoprotein, and prostate specific antigen), viral markers (hepatitis virus and human immunodeficiency virus), and autoimmune antibody titers were all within normal range. Examination for parasites was also negative.
A chest CT scan showed a 2.5-cm diameter mass in the right hilum that changed little in terms of size since 2010 (Figure 1B). A primary lesion was not found in the lung parenchyma. The torso fluorodeoxyglucose (FDG) positron emission tomography identified an abnormal FDG uptake in this right hilar mass, but no other pathologic hypermetabolic lesion was seen that may indicate a primary site. Bronchoscopy revealed mild bronchial wall thickening at the right upper lobe and right middle lobe opening but no endobronchial lesion was present. A biopsy of the bronchial wall thickening was done and histologic examination showed chronic nonspecific inflammation with squamous metaplasia. No abnormal finding was seen on the cytologic exam in the bronchial washing specimen. Endobronchial ultrasound guided transbronchial needle aspiration from the right hilar mass was performed. Atypical cells, favor malignancy, was seen in the aspiration cytology. Endoscopy for gastrointestinal tract and head and neck examination also failed to disclose a primary site of cancer.
Right pneumonectomy and mediastinal lymph node dissection were done. Grossly, several enlarged lymph nodes were found in the hilar and interlobar areas (Figure 2). On serial gross section of the lung, there was no abnormal lesion at bronchial tree and lung parenchyma except multiple lymph nodes enlargement. Entire bronchus and peribronchial tissue were embedded and evaluated. Microscopically, metastatic squamous cell carcinoma was found at several lymph nodes (8/45) and there was no tumor in the lung parenchyma and bronchial tree (Figure 3). Although the tumor was very close to the main bronchus, there was no evidence of bronchial invasion microscopically by tumor. The patient was diagnosed with T0N1M0 lung cancer and has received four cycles of adjuvant chemotherapy with cisplatin and vinorelbine. He has been observed at outpatient clinic after treatment without any evidence of recurrence so far.
CUP is defined as histologically confirmed metastatic cancers whose primary site cannot be identified by standardized diagnostic evaluation at the time of diagnosis14. CUP accounts for about 3% to 5% of all malignancies1,2. Involvement of lymph nodes in the cervical, supraclavicular, and axillary regions with CUP is common, but involvement of intrathoracic lymph nodes such as those in the mediastinal and hilar areas accounted for only 4.2% of lymph node metastases4,15.
CUP is sorted into five major subtypes by microscopic examination. Well or moderately differentiated adenocarcinoma is the most common (60%) histologic subtype, followed by poorly differentiated carcinoma (29%), poorly differentiated malignant neoplasm (5%), squamous cell carcinoma (5%), and neuroendocrine tumor (1%)13,14. Within nodal metastasis range, adenocarcinoma is the dominant subtype expectably except that squamous cell carcinoma is most common in lymph node of head and neck lesion15. Only 12.5%-14.3% of squamous cell carcinoma involve to intrathoracic lymph node, just 4.2% of lymph node metastases with CUP11,15.
Albeit CUP is a heterogeneous group of metastatic malignancy, the majority of patients with CUP present with an aggressive clinical course and poor prognosis with a median survival of 3-9 months and a 12-month survival rate of 24%2,14,15. The gloomy prognosis is caused by CUP's tendency towards early dissemination, with unpredictable metastatic pattern and aggressiveness2,3. But some subsets of CUP present with a favorable prognosis2,3. Lymph node involvement only is one of the subset with good prognosis15. Median survival of single lymph node involvement was 14 months and a 12-month survival rate of 53%. Metastatic squamous cell carcinoma involving the intrathoracic lymph node, like in this case, has a median survival of 16 months and 12-month survival rate of 71%15.
Treatment of CUP should be individualized after considering the clinical information, pathology, involved organ, and other prognostic factors2,14. Generally, intrathoracic lymph node with carcinoma from a CUP is regarded as coming from an occult primary non-small cell lung cancer11. The hypothesis that the primary site of a metastasis into the intrathoracic lymph node from a CUP is of pulmonary origin, is supported by some evidence. First, the lymphatic drainage of the lung passes through the hilar and mediastinal lymph nodes.
These lymph nodes are commonly the first lymph nodes involved in lung cancer metastasis11. Second, most patients with metastatic involvement of the intrathoracic lymph node from a CUP had heavy smoking history, a major risk factor for developing lung cancer11. Because of the above reasons, intrathoracic lymph node with metastasis form a CUP are diagnosed and treated as T0N1M0 or T0N2M0 lung cancer6,7,8,10,12.
A few cases of metastatic squamous cell carcinoma of the intrathoracic lymph node from a CUP have been reported. Reported cases included patients having N1 to N2 lymph node status and all underwent operation (Table 1). The reported cases showed favorable prognosis after complete surgical resection regardless of adjuvant therapy. The patient in this case had a relatively indolent clinical course with the right hilar mass barely changed after 3 years. He had an operation and then received adjuvant chemotherapy. Like the previously reported cases, a favorable prognosis is also expected in this patient.
Metastatic squamous cell carcinoma in the intrathoracic lymph node from a CUP is a very rare subset of CUP. So the best treatment and management for this scarce subset of CUP has not been established, although some literatures have provided information and guidance for physicians caring for patients with CUP. There have been cases of CUP that had complete surgical resection and survived long after the operation. Therefore, surgery is deemed as one of the preferred treatment option for this rare subset of CUP. We reported this rare case and reviewed the associated literatures to help physicians in choosing the best treatment option for these rare cases.
Figures and Tables
 | Figure 1Chest computed tomography (CT) scan in December of 2012 (B) showed a 2.5-cm diameter hilar mass that was little changed, as compared to the cardiac angio CT scan in March of 2010 (A). |
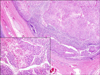 | Figure 3Microscopic finding showed metastatic squamous cell carcinoma to the lymph nodes (H&E stain, ×40; insert image: H&E stain, ×200). |
Table 1
Cases of metastatic squamous cell carcinoma of intrathoracic lymph node from a CUP

| Study | Sex/Age (yr) | Location | Smoking history | Histology | Operation | Adjuvant therapy | Follow-up (mo) | Status |
|---|---|---|---|---|---|---|---|---|
| Morita et al.6 | M/56 | Middle mediastinum (N2) | 26PY | SCC |
Right lower lobectomy LN resection |
None | 20 | No recurrence |
| Yodonawa et al.7 | F/65 | Right mediastinum (N2) | Unknown | SCC | LN resection | RTx | 12 | No recurrence |
| Blanco et al.8 | M/56 | Subcarina (N2) | 50PY | SCC | LN resection | RTx+CTx | Unknown | Under treatment |
| Sakuraba et al.9 | M/63 | Right hilum (N1) | Unknown | SCC | LN resection | None | 43 | Death, SCC on RUL 34 mo after LN resection |
| Kaneko et al.10 | M/63 | Right hilum (N1) | Unknown | SCC |
Right pneumonectomy mediastinal LN dissection |
None | 76 | No recurrence |
| Riquet et al.11 | Unknown | Right hilum (N1) | Heavy smoker | SCC | Right upper lobectomy | RTx+CTx | 8 | Death, vertebral metastasis |
| Tomita et al.12 | M/56 | Left hilum (N1) | 35PY | SCC | LN resection | None | 32 | No recurrence |
References
1. Muir C. Cancer of unknown primary site. Cancer. 1995; 75:1 Suppl. 353–356.
2. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009; 69:271–278.
3. Pavlidis N. Cancer of unknown primary: biological and clinical characteristics. Ann Oncol. 2003; 14:Suppl 3. iii11–iii18.
4. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999; 5:3403–3410.
5. Briasoulis E, Pavlidis N. Cancer of unknown primary origin. Oncologist. 1997; 2:142–152.
6. Morita Y, Yamagishi M, Shijubo N, Nakata H, Kurihara M, Asakawa M. Squamous cell carcinoma of unknown origin in middle mediastinum. Respiration. 1992; 59:344–346.
7. Yodonawa S, Mitsui K, Akaogi E, Onizuka M, Ishikawa S, Kinoshita T, et al. Squamous cell carcinoma of unknown origin affecting mediastinal lymph nodes. Nihon Kyobu Shikkan Gakkai Zasshi. 1996; 34:1364–1368.
8. Blanco N, Kirgan DM, Little AG. Metastatic squamous cell carcinoma of the mediastinum with unknown primary tumor. Chest. 1998; 114:938–940.
9. Sakuraba M, Mae M, Oonuki T, Nitta S. Mediastinal and hilar lymph node of cancer unknown origin: 3 case reports. Nihon Kokyuki Gakkai Zasshi. 1999; 37:72–77.
10. Kaneko K, Yamanda T, Han'uda M, Miyazawa M, Hanaoka T, Kondo R, et al. Metastatic squamous cell carcinoma of hilar lymph node with unknown primary site. Nihon Kokyuki Gakkai Zasshi. 2000; 38:39–44.
11. Riquet M, Badoual C, le Pimpec BF, Dujon A, Danel C. Metastatic thoracic lymph node carcinoma with unknown primary site. Ann Thorac Surg. 2003; 75:244–249.
12. Tomita M, Matsuzaki Y, Shimizu T, Hara M, Ayabe T, Enomoto Y, et al. Squamous cell carcinoma of the hilar lymph node with unknown primary tumor: a case report. Ann Thorac Cardiovasc Surg. 2008; 14:242–245.
13. Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993; 329:257–263.
14. Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G. ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22(Suppl 6):vi64–vi68.
15. Hemminki K, Bevier M, Hemminki A, Sundquist J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann Oncol. 2012; 23:1854–1863.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download