Abstract
Accurate lymph node staging of lung cancer is crucial in determining optimal treatment plans and predicting patient outcome. Currently used lymph node maps have been reconciled to the internationally accepted International Association for the Study of Lung Cancer (IASLC) map published in the seventh edition of TNM classification system of malignant tumours. This article provides computed tomographic illustrations of the IASLC nodal map, to facilitate its application in day-to-day clinical practice in order to increase the appropriate classification in lung cancer staging.
In 2009, the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC) published the seventh edition of the TNM Classification of Malignant Tumors, which was introduced for use in clinical practice from January 1, 2010. The seventh edition of the classification system applies to non-small cell lung cancer (NSCLC), small-cell lung cancer (SCLC)1 and bronchopulmonary carcinoids23. The lymph node map proposed by the International Association for the Study of Lung Cancer (IASLC) was incorporated into the new TNM staging for lung cancer4.
Prior to the publication of the seventh edition, some discrepancies existed in lymph node nomenclature between the two lymph node maps most widely used in clinical practice, the Japanese (Naruke)5 and the Mountain-Dressler modification of the American Thoracic Society map (MD-ATS)6. The most significant difference between these relates to the description of subcarinal lymph node. The new IASLC lymph node map attempted to reconcile the differences between these two lymph node maps and to provide more precise anatomic definitions for all lymph node stations4. This internationally agreed uniform classification system is crucial for treatment planning, for assessing treatment outcomes, and for optimizing future analyses of the prospective international database currently being collected, to facilitate evidence based adjustments for the upcoming eighth edition of the UICC and AJCC staging manuals due to be completed in 2016.
Currently, computed tomography (CT) and positron emission tomography-CT are used routinely for clinical staging of lung cancer, CT having the advantage of greater special resolution and availability. The aim of this article is to review the anatomical definitions of the IASLC lymph node map and provide practical illustration of the mapping system on CT. Moreover, we will give a detailed account of the important changes incorporated in the seventh edition, which affect tumor staging. This simplified illustration of the nodal map provides a useful tool for use in day-to-day clinical practice not only for radiologists but also oncologists, surgeons and pathologists who are involved with the staging and management of patients with lung carcinoma.
The N classification labels the extent of lymph node metastases. The N staging is unchanged on the IASLC map compared with the sixth edition with the N staging validated by survival analysis. The nodal groups by N stage are as follows: N1, ipsilateral intrapulmonary, peribronchial and hilar; N2, ipsilateral mediastinal and subcarinal; and N3, contralateral mediastinal or hilar nodes, ipsilateral or contralateral supraclavicular nodes7. Positive nodes outside the above-defined regions are classified as distant metastasis (M1b).
The anatomic descriptions of each lymph node station have been more precisely described to avoid any overlap in definition between stations. In the IASLC map, the number of lymph node stations is the same as that of sixth edition. However, the 14 stations are reorganized from four groups into seven zones. This new grouping is based on retrospective survival analyses of international databases and lays the foundation for future prospective database centered survival analyses8910.
In the MD-ATS map, the supraclavicular zone lymph nodes were classified as superior mediastinal nodes. However, in the IASLC map station 1 nodes are considered extrathoracic lymph node and all station 1 lymph nodes are classified as N3 disease.
The location descriptions and anatomic boundaries of station 2, 3, and 4 are shown in Table 2 and Figures 2 and 3.
The upper border of stations 2, and 3 is the apex of the lung and pleural space, and in the midline, the upper border of the manubrium. The lower borders of stations 3, 4R, and 4L differ from each other (Figure 2A, B). The lower border of 2R serves as the upper border of 4R, and the same applies for the border between 2L and 4L. For both station 2 and 4, the border between 2R and 2L and between 4R and 4L moves from the midline of the trachea to the left lateral border of the trachea in the IASLC map. This is one of the major changes made in N mapping in the seventh edition (Figure 2C, D).
In the IASLC map definition, the lower border of station 1 is the clavicle bilaterally and, in the midline, the upper border of the manubrium and the upper border of station 2 and 3 extends higher up to the lung apex than the lower border of station 1. Therefore, on axial image, the posterior border of station 1 can abut the anterior border of station 2 or 3p (Figure 4). Because the definitions of the lower border of station 1 nodes are not identical to the definition of the upper border of station 2 and 3 nodes and because the position of the clavicles can vary depending on the level of arm elevation, the inferior-lateral border of station 1 nodes may vary leading to overlap between these nodal groups. The lymph nodes that are closer to the midline are hardly ever affected by the position of the arms11. It would be helpful if the definition of the lower border of station 1 and the upper border of station 2 were identical. This is an improvement, which might be considered for the eighth edition.
The location descriptions and anatomic boundaries of station 5 and 6 are listed in Table 3.
The medial border of station 5 is the ligamentum arteriosum (Figure 5), which is difficult to identify on axial CT unless calcified. The prevalence of ligamentum arteriosum calcification on CT is higher in children (37.8%) than in adults (11.2%) with peak prevalence in 6-10 years, afterwards declining with age12. Although usually invisible on CT the expected location can be estimated by identifying the closest position of the upper pulmonary artery and the aortic arch on coronal reconstructions.
Station 6 lymph nodes lie anterior and lateral to the ascending aorta and aortic arch (Figure 6). The anterior border of station 6 is the imaginary horizontal line extending from the anterior wall of the aortic arch (Figure 6B). In radiological staging it is not clinically important to differentiate stations 5 and 6, both of which lie in the aorto-pulmonary nodal region and differentiation does not affect staging.
The location description and anatomic boundary of station 7 is listed in Table 4.
Station 7 encompasses a larger area in the new seventh edition IASLC map than on previous map versions and is most easily identified on the coronal images (Figure 7A). This is one of the major changes and reconciles the Naruke and MD-ATS maps11.
The nodes located in the space between the medial margins of both main bronchi are station 7 and nodes outside of the space are station 10 (Figure 7B)413. Below the carina of the trachea, the nodes in the posterior mediastinum are station 7 and above this level 3p. Within the cranio-caudal boundary of the subcarinal zone, the posterior margin of station 7 extends posteriorly to the paraesophageal area at this level (Figure 7B) with station 8 upper boundary below this level. Station 7 nodes are considered ipsilateral (N2) with the primary tumor irrespective of side.
The location descriptions and anatomic boundaries of station 8 and 9 are listed in Table 5.
Below the lower border of subcarinal zone, station 8 nodes lay adjacent to the wall of the esophagus and to the right or left of the midline (Figure 8).
Station 9 nodes are located within the inferior pulmonary ligament (Figure 9) and separation of station 8 and 9 is easy at surgery. In the region of the inferior pulmonary vein, the mediastinal and visceral pleura merge to form the inferior pulmonary ligament. It extends downwards toward to the hemidiaphragm anchoring the lower lobes to the mediastinum. The length of this ligament may vary both from person to person and in the same individual1415.
The location descriptions and anatomic boundary of station 10 and 11 are listed in Tables 6 and 7, respectively.
Station 10 includes the nodes immediately adjacent to the mainstem bronchus and hilar vessels such as the proximal portions of the pulmonary veins and main pulmonary arteries. There is a change of the borders between station 4 and 10 with the pleural reflection no longer serving as the border between them (Figure 10)4. The lymph nodes around the both main bronchi within the mediastinum are considered as station 10; the nodes below the lower margin of the azygos vein as 10R, and the nodes below the upper rim of the left main pulmonary artery as 10L. Therefore, some tumors previously staged as N2 are now staged as N1. Pitson et al.16 and El-Sharief et al.17 discussed a potential ambiguity for lymph nodes at the level of the tracheal bifurcation below the level of the superior vena cava-azygos vein junction, and grouped lymph nodes anterior to the tracheal bifurcation with the lower paratracheal station17. However, Lee et al.18 reported that the changed definition between N1 and N2 diseases by IASLC map works well in classifying patient prognosis.
Station 10 also has a common boundary with station 7 and border is a vertical line from the medial margin of the both main bronchi (Figure 7B).
Station 12 is a node adjacent to the lobar bronchi. Station 13 is a node adjacent to the segmental bronchi. Station 14 is a node adjacent to the subsegmental bronchi. These nodes are intrapulmonary and for staging purposes are to be differentiated from intrapulmonary metastasis. Differentiation affects staging as an intrapulmonary metastasis in the same lobe as the primary tumour would be a T3 cancer while station 12, 13, or 14 nodes would be N1 disease. Nodes are usually peribronchovasular often at areas of bronchial branching.
The seventh edition IASLC map was formulated after retrospective analysis of outcome data for a multinational database. The seventh edition IASLC map therefore provided more precise anatomic definitions than that of sixth edition, however there remain several outstanding areas of uncertainty, which could be addressed, in later editions.
(1) There is no specific definition of the border between right and left side for station 3a, 3p, and 8, which is necessary to classify N2 or N3 tumour. It is unclear whether this should be the midline or the left side of the trachea as when categorising station 2 and 4 nodes.
(2) Some lymph nodes in the thorax, which lung cancer only occasionally metastasises to, are not included in the IASLC lymph node map at all. These include anterior, middle, and posterior diaphragmatic nodes, intercostal nodes, internal mammary node, retrocrural nodes and axillary node. It is unclear whether these should be classed as N2 or N3 disease.
(3) The IASLC classification doesn't relate the relationship between overall survival and lymph node burden addressing issues such as single metastases versus multiple metastases, the significance of skip metastases and extranodal tumour spread.
Uniform application of the IASLC map will provide the database for future prospective analysis to solve the ambiguities and to make more complete lymph node classification.
The seventh TNM staging and IASLC map are applied to NSCLC, SCLC, and bronchopulmonary carcinoids.
The major changes in the IASLC nodal map are as follows: (1) border between station 2R and 2L moves from the midline of trachea to the left lateral wall of the trachea (Figure 2C, D); (2) border between station 4R and 4L moves from the midline of trachea to the left lateral wall of the trachea (Figure 2C, D); (3) station 7 encompasses a larger area in the IASLC map and this reflects reconciliation between the Naruke and MD-ATS maps (Figure 7); (4) there is a change of the borders between station 4 and 10 with the pleural reflection no longer serving as the border between the two stations (Figure 10).
Figures and Tables
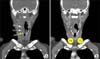 | Figure 1Station 1. (A) The upper border of station 1 is the lower margin of the cricoid cartilage (yellow arrow). The lower border is clavicles bilaterally (C), in the midline, the upper border of the manubrium (M). (B) The border between 1R and 1L is the midline of the trachea (dashed line). C: clavicle; CR: cricoid cartilage; HY: hyoid bone; M: manubrium; TH: thyroid cartilage. |
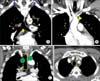 | Figure 2Upper zone: stations 2R, 2L, 3a, 3p, 4R, and 4L. (A, B) Upper and lower borders of stations 2, 3 and 4. The upper border of station 2R, 2L, 3a and 3p is the apex of each lung and pleural space (blue arrows), and in the midline, the upper border of the manubrium. The lower borders of the stations are different from station to station: station 2R: the intersection of caudal margin of innominate vein with the trachea (yellow triangle in B); station 2L: the superior border of the aortic arch (yellow line); station 3a and 3p, the level of carina (yellow arrow); station 4R: the lower border of the azygos vein (red line); station 4L: the upper rim of the left main pulmonary artery (yellow dashed line). (C, D) The border between 2R and 2L and between 4R and 4L moves from the midline of trachea to the left lateral border of the trachea. AA: aortic arch; AZ: azygos vein; LIV: left innominate vein; PA: left main pulmonary artery; T: trachea. |
 | Figure 3Stations 3a and 3p. (A) The upper border of station 3a and 3p is the apex of chest (blue arrows) and lower border is the level of carina (yellow arrow). (B) The anterior border of 3a is the posterior aspect of the sternum, and the posterior border is the anterior border of the superior vena cava on the right and left carotid artery on the left. Station 3p is a retrotracheal lymph node. LCA: left common carotid artery; LIV: left innominate vein; SVC: superior vena cava. |
 | Figure 4Boundary distinction among stations 1, 2 and 3. (A) Station 1 supaclavicular lymph node. (B) Inferior to the lung apex, the yellow line running horizontally from anterior margin of both lung pleura interface separates station 1 from station 3p. (C) At the suprasternal notch, the yellow line running horizontally from anterior margin of both lung pleura interface separates station 1 from station 2. The red line running along posterior wall of trachea separates station 2 from 3p. (D) Station 3a and 3p at the level of tracheal bifurcation (dashed red arrow). |
 | Figure 5Station 5. (A) The upper border of station 5 is the lower border of aortic arch (AoA), and lower border is the upper rim of the left main pulmonary artery (PA). (B) The border between station 4L and station 5 is the ligamentum arteriosum (sky-blue arrow). Station 4L nodes locate medial to the ligamentum arteriosum (blue arrow), and station 5 is the lymph node lateral to the ligamentum arteriosum (brown arrow). AA: ascending aorta; AZ: azygos vein; DA: descending aorta. |
 | Figure 6Station 6. (A) The upper border of station 6 is a line tangential to the upper border of the aortic arch and lower border is the lower border of aortic arch (green lines). (B) The anterior border of station 6 is the imaginary horizontal line extending from the anterior wall of the aortic arch (yellow line), which discriminates station 6 from station 3a. AoA: aortic arch. |
 | Figure 7Subcarinal zone: Station 7. (A) Upper and lower borders of station 7 are well identified on coronal image. (B) Station 7 nodes are noted in the space between the medial margin of both main bronchi (yellow lines) and nodes outside of the space are station 10. Station 7 extends posteriorly around the esophagus. AoA: aortic arch; BI: bronchus intermedius; LLL: left lower lobe; LMB: left main bronchus; RMB: right main bronchus; RUL: right upper lobe. |
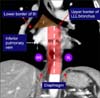 | Figure 8Lower zone: stations 8 and 9. The upper border of station 8 is the upper border of the left lower lobe (LLL) bronchus on the left side and the lower border of the bronchus intermedius (BI) on the right side. The upper border of the station 9 is the inferior pulmonary vein. The lower border of station 8 and 9 is the diaphragm. The border between 8R and 8L is the midline (dashed line). |
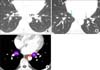 | Figure 9Station 9 and the inferior pulmonary ligament. (A) Both inferior pulmonary veins joining the left atrium can be easily identified on computed tomography images. (B) Right and left inferior pulmonary ligaments (sky-blue dashed arrows) attach each lower lobe to the diaphragm. (C) Stations 8 and 9 nodes on axial image. Station 9 nodes locate in the inferior pulmonary ligament. Station 8 nodes are present adjacent to the esophagus (black circle). LIPV: left inferior pulmonary vein; RIPV: right inferior pulmonary vein. |
 | Figure 10Station 10. (A) The border between station 4R and 10R is the lower rim of the azygos vein (red dashed arrow). The border between station 4L and 10L is the upper rim of the left main pulmonary artery (red line). (B) Station 4R and 4L nodes at the level of azygos vein. Note the border between station 4R and 4L is the left lateral wall of the trachea (yellow line). (C) Stations 10R and 10L below the azygos vein. The pleural reflection (pink arrowhead) no longer serves as the border between station 4 and 10. The border between station 10R and 10L is the midline of tracheal bifurcation (yellow line). AoA: aortic arch; AZ: azygos vein; PA: left pulmonary artery. |
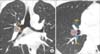 | Figure 11Station 11R. (A) Station 11Rs is located between the right upper lobe bronchus and bronchus intermedius. (B) Station 11Ri is present between the right middle and lower lobe bronchi. Lung window setting is useful to define the bronchial details. BI: bronchus intermedius; RLL: right lower lobe; RML: right middle lobe; RUL: right upper lobe. |
Table 1
Anatomic boundary of station 1

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 2
Anatomic boundaries of Station 2, 3, and 4 in the upper zone

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 3
Anatomic boundaries of stations 5 and 6

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 4
Anatomic boundary of station 7

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 5
Anatomic boundary of stations 8 and 9

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 6
Anatomic boundary of station 10

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
Table 7
Anatomic definition of station 11

Adapted from Rusch VW et al. J Thorac Oncol 2009;4:568-77, with permission4.
References
1. Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM edition. J Thorac Oncol. 2009; 4:300–310.
2. Greaves SM, Brown K, Garon EB, Garon BL. The new staging system for lung cancer: imaging and clinical implications. J Thorac Imaging. 2011; 26:119–131.
3. Travis WD, Giroux DJ, Chansky K, Crowley J, Asamura H, Brambilla E, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2008; 3:1213–1223.
4. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009; 4:568–577.
5. Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. 1978; 76:832–839.
6. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997; 111:1718–1723.
7. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012; 4:128–134.
8. Marshall HM, Leong SC, Bowman RV, Yang IA, Fong KM. The science behind the 7th edition Tumour, Node, Metastasis staging system for lung cancer. Respirology. 2012; 17:247–260.
9. Takamochi K, Oh S, Suzuki K. Prognostic evaluation of nodal staging based on the new IASLC lymph node map for lung cancer. Thorac Cardiovasc Surg. 2010; 58:345–349.
10. Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007; 2:603–612.
11. Rusch VW, Asamura H. Response: a critique of the international association for the study of lung cancer lymph node map. J Thorac Oncol. 2012; 7:481.
12. Hong GS, Goo HW, Song JW. Prevalence of ligamentum arteriosum calcification on multi-section spiral CT and digital radiography. Int J Cardiovasc Imaging. 2012; 28:Suppl 1. 61–67.
13. Lynch R, Pitson G, Ball D, Claude L, Sarrut D. Computed tomographic atlas for the new international lymph node map for lung cancer: a radiation oncologist perspective. Pract Radiat Oncol. 2013; 3:54–66.
14. Cooper C, Moss AA, Buy JN, Stark DD. CT appearance of the normal inferior pulmonary ligament. AJR Am J Roentgenol. 1983; 141:237–240.
15. Rost RC Jr, Proto AV. Inferior pulmonary ligament: computed tomographic appearance. Radiology. 1983; 148:479–483.
16. Pitson G, Lynch R, Claude L, Sarrut D. A critique of the international association for the study of lung cancer lymph node map: a radiation oncology perspective. J Thorac Oncol. 2012; 7:478–480.
17. El-Sherief AH, Lau CT, Wu CC, Drake RL, Abbott GF, Rice TW. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics. 2014; 34:1680–1691.
18. Lee S, Lee HY, Lee KS, Yie M, Zo J, Shim YM, et al. Change of junctions between stations 10 and 4 in the new international association for the study of lung cancer lymph node map: a validation study from a single, tertiary referral hospital experience. Chest. 2015; 147:1299–1306.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download