Abstract
Bronchopulmonary dysplasia (BPD) is related to decreased lung function throughout life. However, the pathology and radiology pattern of BPD of adults are not documented well yet. In this case report, we present BPD case of an adult monozygotic twin showing nearly identical lesions on chest computed tomography (CT). CT images showed mixed areas of ground-glass and reticular opacities in both lungs. They had common histories of pneumonias requiring mechanical ventilations in period of infants. Pulmonary function test of one patient showed a pulmonary insufficiency with airway obstruction. Pathologic findings showed bronchiolar hyperplasia and peribronchiolar fibrosis which was similar to classic BPD patients. Our twin case report might help provide distinguishing pathology and radiology pattern of an adult pulmonary sequelaes of BPD. It might be reasonable to make close follow-up for BPD patients to evaluate the long-term outcomes of BPD survivors.
Bronchopulmonary dysplasia (BPD) still remains a leading cause of morbidity, mortality and long-term sequelae of premature infants1. Although BPD developed in the neonatal period, BPD patients tend to have consistently greater incidences of respiratory morbidity, hospitalization, and long-term pulmonary impairments such as asthma, emphysema and pulmonary hypertension2,3,4. The main risk factors evolving to BPD include prematurity, mechanical ventilation, oxygen administration and infection5. As twins studies have suggested that unknown genetic factors are also the major contributive factors for development of BPD, there were increased interests in the hereditability of BPD, however it is still unclear how it develops6,7. In this case report, we are keen to present the cases of an adult monozygotic twin that showed nearly identical damaged lesions of lung on a chest computed tomography (CT), which were not the common radiologic findings of the pulmonary sequelae of BPD. They had a common medical history of prematurity and episodes of mechanical ventilation. As well as, an obtained lung tissue by video-assisted thoracic surgery (VATS) wedge resection enabled us to observe the rare pathological findings of BPD in adult period. This twin case report may suggest some important clues about long-term prognosis, clinical presentations, and pathology of BPD in adults.
A 29-year-old male visited our center complaining of cough, sputum and coryza. These respiratory symptoms developed about 20 days prior to visit, and the patient has been prescribed oral medications including cefditoren and azithromycin within the preceding 10 days at local clinic. The patient was admitted to our center by the end of December 2013, as respiratory symptoms persisted despite medications. He had no significant past medical history, except a history of pneumonia during neonatal period, receiving treatment including mechanical ventilation. He was never-smoker and is working in office. He had no regular medications and only took recently prescribed medications for respiratory symptoms.
At the time of admission, the patient's vital signs, including body temperature, pulse rate, blood pressure, respiratory rate were 36.6℃, 108 per minute, 148/84 mm Hg, and 20 per minute, respectively. On a physical examination, he showed a symmetric expansion of the thorax related to respiration, with slight crackle on the right middle lung field. On a routine blood test, white blood cell count was 8,640/µL (neutrophil 62.5%, lymphocyte 26.2%), and C-related petide level was 0.17 mg/dL. There was no significant abnormality otherwise, except mild elevation of alanine transaminase (ALT) (aspartate transaminase/ALT level, 39/70 IU/L). After admission, patient's pulmonary function test (PFT) was performed. It showed forced vital capacity (FVC) of 3.87 L, forced expiratory volume in 1 second (FEV1) of 3.26 L, which were 74%, 76% of predicted value, respectively. Diffusing capacity for carbon monoxide was 17.7 mL/mm Hg/min, 57% of predictive value. A reversible bronchodilator response was not seen at this time. His forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) was 3.78 L, and it was 86% of the predicted value.
The patient's chest posteroanterior radiograph showed symmetrically increased peribronchovascular opacities in both lungs. He underwent a low-dose CT scan (120 kVp and 30 mAs; 2-mm slice thickness) of the thorax, and CT images showed mixed areas of ground-glass and reticular opacities in both lungs, predominantly along central and peribronchovascular areas (Figure 1). The patient was admitted for diagnosis and treatment as the chest CT findings suggested the possibility of interstitial lung disease. For definitive diagnosis, VATS wedge resection for tissue confirm was scheduled and several serologic tests for viral and atypical pathogens were also performed. Additionally, antibiotics were switched to intravenous piperacillin-tazobactam, and steroid therapy with methylprednisolone (32.5 mg intravenous Q12hr) was also administrated to improve respiratory symptoms and radiologic findings. VATS was performed on fourth day of admission. The superior segment of left lower lobe was resected with visual identification of the consolidation.
Pathological examination of the lung specimen showed accentuated bronchioles due to bronchiolar hyperplasia with cystic dilatation of bronchiolar lumens, reminiscent of congenital pulmonary airway malformation. Also, peribronchiolar fibrosis was present, as well as smooth muscle hyperplasia. Alveolar structures were relatively intact except for focal emphysematous change. These pathologic findings were similar pattern to classic BPD. Some bronchiolar lumens were filled with necro-inflammatory mucoid material, suggestive of superimposed recent bronchiolitis (Figure 2). These findings suggest previous small airway damage, accompanied by recent bronchiolitis. The possibility of cryptogenic organizing pneumonia or other atypical pneumonia could be ruled out by pathologic findings.
For the follow-up evaluation, low dose chest CT follow-up was performed on 12th day of admission, but it showed no significant interval change after medical treatment with antibiotics and steroid. PFT was also followed up on the same day, and FVC and FEV1 equally showed 36% of predicted value but post-bronchodilator FEV1 was 47% of predicted value, increasing about 31% after bronchodilator inhalation. He complained a chest pain which could be explained the result of his lower FEV1 compared with preoperative basal FEV1 but the reversibility after bronchodilator inhalation may also suggest his potential asthmatic component. With the results of radiology, pathology and the patient's past medical history, the patient's damaged lung lesions might be assumed to be sequelae of BPD. As the patient's symptoms had subsided, he was discharged on 17th day of hospitalization, and planned to follow-up on out-patient department for asthma evaluation and treatment.
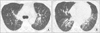
Meanwhile, 29-year-old male, the twin brother of the abovementioned patient, visited our hospital for further evaluation after his brother discharge, concerning about abnormal findings found in his sibling. A low-dose chest CT was performed on an out-patient department, and the chest CT showed peribronchovascular mixed ground-glass and reticular opacities in both lungs, nearly identical lesions to the chest CT findings of the patient's twin brother (Figure 3).
Despite of advances in medical treatment for several decades, BPD is still the most common cause of chronic lung disease during infancy8. As the survival rates for extremely preterm neonates have increased significantly with the help of recent improved medical treatments, concerns about long-term outcome for BPD survivors have been raised. BPD survivors show similar pulmonary sequelae on radiographic findings3 and common compromised pulmonary functions 9,10,11. Our patients were, indeed, at a high risk to have BPD sequelae because of preterm birth and history of mechanical ventilation. However, they showed some unique features distinct from other BPD survivors in view of radiologic findings and histologic feature. The most common findings of BPD radiologic features in children and young adults are linear opacities, triangular opacities, and air trapping, which appears in up to 70% of BPD survivors12. The usual findings of chest radiographs of BPD in adults have been demonstrated reduced lung attenuation, bronchial wall thickening, linear opacities, bullae and decreased bronchus to pulmonary artery diameter ratio. Howling et al.3 demonstrated that bronchus to pulmonary artery diameter ratio was 0.95±0.08 in healthy subjects and 0.45±0.04 (p<0.01) in patients with BPD survivors. Decreased bronchoarterial diameters imply reduced bronchial diameters rather than increased pulmonary artery diameters. The chest CT finding of our patient showed mixed groundglass and peribronchovascular reticular opacities in both lungs. These features may be similar with the radiologic findings of interstitial pneumonia. The radiologic lesions of our patients may have resulted from recent respiratory infections, considering BPD survivors tend to have more frequent respiratory infections13. Actually, one of twin brother had respiratory symptoms for a month. This atypical findings of chest CT, which is not very common in other BPD survivors, may suggest the combined acute inflammation. However, there is no proper explanation concerning the nearly identical findings from his twin sibling, who had no respiratory symptoms. Probably some unknown factors, especially genetic polymorphism may be operated in developing of BPD. It is well known that BPD survivors tend to have a variety of pulmonary function abnormalities including decreased pulmonary diffusion capacity, decreased small airway patency, and lower FEV1 and FEF25-75. Landry et al.11 reported that the values of FEV1, FVC, and FEF25-75 in 322 preterm infants were significantly associated with the initial BPD severity. The PFT findings of our patient showed similar pattern of pulmonary insufficiency with reversible airway obstruction after VATS. The EPICure study by Fawke et al.14 demostrated that about 25% of their cohort of children born less than 26 weeks gestation had been diagnosed as asthma. Our patient showed no responses on bronchodilator at the initial PFT after admission, but substantial bronchodilator response was noted on postoperative PFT. Although this result may have been influenced by operation site pain since PFT was performed on a week later after operation, the patient may intrinsically had asthmatic component. Histologic findings in BPD divide by classic BPD and new BPD. Classic BPD, which was prior to utilization of surfactant replacement therapy was characterized by lung inflammation, airway injury, and parenchyma fibrosis due to hyperinflation. The histologic features of our patient showed similar classic BPD. The new BPD patients have less inflammation and fibrosis including alveolar arrest, reduction in distal alveolar growth, and a reduced incidence of fibroproliferation15. As the BPD survivor's lungs are likely to have been damaged to a varying extent, it is assumed that pathological findings would also vary. Our patient showed dominant pathological changes on small airways. He showed bronchiolar hyperplasia with cystic dilatation of bronchiolar lumens, and somewhat emphysematous change of alveolus. These kinds of cases are rare, in which pathological findings are obtained from clinically suspected BPD patients in adult period.
One of the most unique features of our case was that twin sibling showed very similar features on their chest CT scans, and it seemed likely genetic factors would have attributed to this phenomenon, as they had the similar lesions even with their different presentations. Since the twin study by Bhandari et al.6 showed that genetic factors accounted for 53% of the variance in liability for BPD. Genetic attribution on BPD got to the attention and further studies, accordingly.
In summary, we presented twin patients who had pneumonias during the neonatal period, and required mechanical ventilations, showing the very similar lesions suggesting BPD on their chest CT. One of our patients showed prolonged respiratory symptoms over 2 weeks and significant pulmonary insufficiency implying obstructive pattern on his PFT. Although the majority of BPD survivors only show subtle abnormalities on their chest radiographs without significant respiratory symptoms, they have a relative higher risk to develop to asthma or other chronic obstructive lung disease. It is reasonable to pay more attention on following up the BPD survivors, especially when they have twin sibling suffering pulmonary morbidities.
Figures and Tables
Figure 1
A low dose chest computed tomographic (CT) findings of 29-year-old man. (A) Posterior-anterior chest radiograph showing peribronchovascular increased opacities in both lungs. (B, C) CT scans (2-mm slice low-dose CT; lung window images with window level of-700 Hounsfield unit (HU) and window width of 1,500 HU) showing areas of mixed ground-glass and reticular opacities in both lungs, predominantly in the central and peribronchovascular areas.

Figure 2
Pathologic findings of a patient who had undergone video-assisted thoracic surgery. (A) Lower power field lesions showing bronchiolocentric distribution. Alveolar area is relatively intact (×10). (B) Bronchiolar area showing bronchiolar hyperplasia with cystic dilatation and peribronchiolar fibrosis (×40). (C) Peribronchiolar fibrosis and smooth muscle hyperplasia are noted in dilated bronchioles. Bronchiolar lumen is lined by bronchiolar typed epithelium (×100). (D) Subpleural fibrosis and septal fibrosis aggregation of alveolar macrophages are noted (×40).
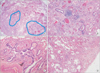
Figure 3
A 29-year-old man who is the monozygotic twin of the person in the case of Figure 1. (A, B) Low dose computed tomograpy (CT) scans showing peribronchovascular mixed ground-glass and reticular opacities in both lungs, which had a nearly identical pattern as the chest CT findings of his twin.
References
1. Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006; 117(3 Pt 2):S52–S56.
2. Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013; 37:132–137.
3. Howling SJ, Northway WH Jr, Hansell DM, Moss RB, Ward S, Muller NL. Pulmonary sequelae of bronchopulmonary dysplasia survivors: high-resolution CT findings. AJR Am J Roentgenol. 2000; 174:1323–1326.
4. Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008; 32:321–328.
5. Bancalari E. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol. 2001; 18:1–9.
6. Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006; 117:1901–1906.
7. Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008; 122:479–485.
8. Bhandari A, Bhandari V. "New" bronchopulmonary dysplasia: a clinical review. Clin Pulm Med. 2011; 18:137–143.
9. Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006; 173:890–896.
10. Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr. 2007; 150:256–261.
11. Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011; 18:265–270.
12. Aukland SM, Halvorsen T, Fosse KR, Daltveit AK, Rosendahl K. High-resolution CT of the chest in children and young adults who were born prematurely: findings in a populationbased study. AJR Am J Roentgenol. 2006; 187:1012–1018.
13. Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006; 30:219–226.
14. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010; 182:237–245.
15. Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006; 30:179–184.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download