Abstract
MicroRNAs (miRNAs) are a class of small noncoding RNAs that modulate target gene activity, and are aberrantly expressed in most types of cancer as well in lung cancer. A miRNA can potentially target a diverse set of mRNAs; further, it plays a critical role in lung tumorigenesis as well as affects patient outcome. Previous studies focused mainly on abnormal miRNAs expressions in lung cancer tissues. Interestingly, circulating miRNAs were identified in human plasma and serum in 2008. Since then, considerable effort has been directed to the study of circulating miRNAs as one of the biomarkers of lung cancer. miRNAs expression of tissues and blood in lung cancer patients is being analyzed by more researchers. Recently, to overcome the high false-positivity of low-dose chest computed tomography scan, miRNAs in lung cancer screening are being investigated. This article summarizes the recent researches regarding clinical applications of miRNAs in the diagnosis and management of lung cancer.
Technological advances have enhanced the diagnosis and treatment of lung cancer, but the mortality is still high. Lung cancer is hardly detected at early curable stage and there is lack of effective antitumor agents that induce few adverse effects, except for some target therapies. What we need is developing a hematologic marker for lung cancer and a more effective therapy than conventional ones. Efforts are being made to achieve these goals and studies using microRNA (miRNA) have gained a lot of attention recently, ranging from those on basic pathogenesis to those on actual clinical applications. This study was aimed at investigating how far those miRNA researches have progressed with regard to lung cancer and how useful miRNA is in clinical practice.
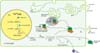
miRNA is a single-stranded non-coding RNA molecule, containing 18-25 nucleotides, and is responsible for the expression of various genes in eukaryotes. It has been reported that it is involved in controlling various functions, such as cell development, differentiation, proliferation, cell death, stress reaction, fat metabolism, insulin secretion and carcinogenesis. miRNA combines complementarily with 3' untranslated region (3' UTR) of target genes to control those target genes by inhibiting mRNA translation. Single miRNA may affect multiple mRNAs or multiple miRNAs may affect single mRNA. It is well known that miRNA is involved in a lot of biological processes of normal cells, and accumulating data suggests expression of abnormal miRNAs in many diseases. The process of miRNA biogenesis is presented in Figure 11.
Details of the biogenesis process are not discussed here in order to focus on clinical features of miRNA.
It has been reported that abnormal miRNAs are also detected in hematologic malignancies and solid cancers. For example, miR-15 and miR-16 combine with B cell lymphoma 2 (BCL2) to induce apoptosis in normal cells but their expression is reduced in malignant lymphoma, possibly because cell apoptosis is inhibited by abnormal expression of miRNAs. In addition, miRNA is thought to be closely associated with signaling in cancer development and cancer metastasis. With studies revealing interactions between miRNA and previously known cancer genes, recent data suggests the role of miRNA in cancer stem cells2.
Abnormal expression of miRNA in lung cancer has been researched since 2004, demonstrating interaction between miRNA and various lung cancer-related genes (Table 1). One example is the interaction between RAS and p53 genes and the expression of miRNAs. RAS mutation (K-RAS, especially) is clinically important in lung cancer and the let-7 family of miRNAs is known to regulate RAS expression. In 2005, Johnson et al.3 reported that let-7 regulated Caenorhabditis elegans let-60 gene (the ortholog of human RAS) and that 3' UTR of human RAS gene had a lot of let-7 complimentary sites. They confirmed that RAS expression was increased when let-7 was inhibited in normal lung epithelial cell. In tumor tissues from lung cancer, let-7 was reduced at the same time when RAS expression was increased. Researches later found associations between various miRNAs, including miR-451, and RAS gene. There have been studies about miRNAs which is related to p53, a representative tumor suppressor. According to results from studies using a lung cancer stem cell (H1299), p53 increased the expression of miR-34a, inhibiting the proliferation of lung cancer cells and facilitating apoptosis. There is evidence supporting the association between p53 and various miRNAs, including miR-125a. Accumulating data also suggests complex interactions between a lot of cancer genes and signaling molecules4.
A number of studies have reported miRNA detection in tumor tissue as a diagnostic and prognostic indicator of cancer. Results so far have been promising, although there is some controversy over its prognostic value. With recent identification of circulating ones, miRNA is considered as a possible noninvasive tumor marker. It is clinically promising as a tumor marker because it is stable in patient's tissues or fluids, unlike typical RNAs. miRNA is stably detected in frozen tissues as well as in formalin fixed paraffin embedded tissues, serum, sputum and pleural effusion, mostly by means of microarray or quantitative reverse transcriptase polymerase chain reaction. The short sequence is also beneficial for lowering the cost of analysis.
Earlier studies had used tumor tissues from lung cancer and normal lung tissues, but the identification of circulating miRNA has made later studies using body fluids (blood, sputum, pleural effusion, etc.) as well tissues in lung cancer patients. Several researchers found diverse miRNAs being detected in a stable way, but most of the studies were retrospective, and further results are required before using them for routine diagnosis. The association between abnormal expression of miRNA and lung cancer was first reported in 2004. Takamizawa et al.5 measured the level of let-7 expression in normal and tumor tissues from surgically removed lungs in lung cancer patients and found that the expression of let-7 was reduced in tumor tissues compared to that in adjacent normal lung tissues. The survival was shorter in patients with relatively lower level of let-7. Multivariate analysis showed this prognostic impact to be independent of disease stage, age, sex and smoking status. Researches later found miRNAs in various types of patients and attempted to determine its clinical significance. In 2008, Yu et al.6 reported a five-miRNA (has-let-7a, has-miR-221, has-miR-137, has-miR-372 and has-miR-182) signature as a prognostic marker in 112 patients with non-small cell lung cancer (NSCLC). Studies are also investigating miRNA for its predictive value of treatment response. For example, miR-22 expression varied among patients with progressive lung cancer according to their response to pemetrexed therapy7. A study even suggested that the genetic polymorphism of a specific miRNA was a prognostic marker8. More studies are expected to contribute to the data on miRNA in a similar way. miRNAs have been detected in pleural effusion as well as in blood in lung cancer patients. The pattern of miRNA expression in pleural effusion was associated with the patient's prognosis of lung cancer in some cases. More specifically, higher expression of miR-100 and the lower the expression of miR-93, miR-134, miR-151, and miR-345, showed the tendency of poor prognosis9.
It seems still premature to recognize miRNA as a prognostic factor, because investigators reported conflicting data on the prognostic value of the same miRNA. In a large-scale analysis of samples obtained from International Adjuvant Lung Cancer Trial (IALT), miRNAs that had been found as significant predictors of prognosis (miR-21, miR-29b, miR-34a/b/c, miR-155, and let-7a) were no longer significant10. It might be due to the heterogeneity of lung cancer. Lung cancer varies in clinical presentation depending on the histology and genetic mutation. So, specific miRNAs are reported to be expressed differently according to the histology of lung cancer11. Results of miRNAs expression may be helpful for histological diagnosis of NSCLC. However, this situation suggests that diverse factors such as histologic subtypes of lung cancer must be considered to predict prognosis with miRNAs. miRNA profile was different between primary cancer and metastatic cancer and between smoker and nonsmoker12,13. Further prospective studies are required in selected population to determine the prognostic value of miRNA.
In addition to the attempts to use circulating miRNA as a diagnostic tool, the possibility of using it for early diagnosis has been considered as well. An analysis showed differential expression of serum miRNAs (miR-155, miR-182, and miR-197) between cancer patients and normal population or patients with infectious diseases (pneumonia or tuberculosis)14. It would be clinically important, therefore, if a miRNA expression before diagnosis or at early stage of cancer could be presented in any way as an evidence of lung cancer. It would be helpful when determining whether or not to perform invasive biopsy if serum miRNA analysis could be used for determining malignancy of solitary pulmonary nodules15. Recent studies tend to use statistical software to identify individuals who have a high risk of lung cancer, by conducting a pattern analysis among a large number of miRNAs. In a recent analysis of 34 circulating miRNAs, the diagnostic accuracy of early lung cancer was approximately 80% among asymptomatic high-risk individuals16.
miRNA may have a high specificity depending on the tissue or disease, but care should be made before suspecting and making a diagnosis of lung cancer entirely based on miRNA expression. First we need to fill in the gaps in miRNA expression among a wide range of other diseases. One possibility is to consider miRNA as a supplement to conventional tests. Low-dose chest computed tomography (CT) is currently used for early lung cancer screening among high-risk individuals with smoking history. This strategy was recently found to reduce lung cancer mortality in a large study, but false-positive rate was too high. Recently, it has been suggested that circulating miRNA would be useful for lowering the false-positive rate. Based on high specificity of circulating miRNA for lung cancer, it would be possible to use it as a complement to the low-dose chest CT. One example of this is a large study (MILD trial) that was conducted in Italy since 2005. In addition to assessing the usefulness of low-dose chest CT for lung cancer screening, the investigators also obtained blood samples from the subjects and analyzed them for 24 miRNAs. miRNA analysis alone had 87% sensitivity and 81% specificity for lung cancer diagnosis. Negative predictive value was as high as 99%. On the contrary, the rates were 79%, 81%, and 19.4%, respectively, for low-dose chest CT screening. When low-dose chest CT and miRNA were combined, false positive rate was significantly reduced to 3.7%17. This is supporting the evidence that miRNA could contribute to the early diagnosis of lung cancer.
Shen et al.18 found 10 miRNAs which were expressed differently in the sputum of lung cancer patients than in normal individuals. Among them, miR-31 and miR-210 showed 65.2% sensitivity and 89.7% specificity for lung cancer diagnosis. When they were combined with chest CT, specificity was elevated to 91.2%18. It is expected that these attempts to use miRNA for early diagnosis of lung cancer in the clinical practice will continue in the future.
Imaging technology of a particular molecule change in cancer patients is now being studied. Study on miRNA combined with fluorescence-based imaging and bioluminescence-based imaging using luciferase for detection of its change is currently in the stage of animal testing and is considered to be useful for lung cancer diagnosis and evaluation of pre- and post-treatment reactions. Combination with nanotechnology is necessary to develop a vector with in vivo safety.
Researches on increasing or decreasing miRNAs in various stages of lung cancer development are now ongoing in animal study. These studies are focusing not only on directly destroying tumor itself but also on the modulation of tolerance to conventional therapies. miRNAs are used to determine the mechanism of developing tolerance to cytotoxic chemotherapies and target therapies. It has been reported that miRNAs are associated with several genes that are related to such tolerance (excision repair cross-complementation group 1, for example). Sensitivity to chemotherapy can be recovered by controlling such miRNAs. Association between miRNAs (such as let-7 and miR-31) and genes related to radiotherapy sensitivity has been also been proving, most of them being in vitro studies. It is expected that controlling these miRNAs would be useful for increasing the effectiveness of radiotherapy. There is accumulating data suggesting that miRNAs could be used to decrease metastasis, if not to destroy tumors directly, and successful results have been achieved in cancer stem cells. In addition to directly controlling miRNAs, controlling miRNA expression by using new materials and combination with conventional chemotherapies are now being studied as well. More follow-up studies are needed to apply miRNA for treatments in clinical practice.
In lung cancer diagnosis, histological or genetic analysis may be limited by small sample size. Moreover, there is a lack of good biomarkers available for the prediction of prognosis. Low-dose chest CT for early lung cancer screening is limited by high false positive rate and the risk of radiation exposure for general application. In this context, miRNA has been investigated to overcome these issues and some of the studies have reported promising results. It may be still premature to apply miRNA in clinical therapies but it will not be long before it could be used for diagnostic purposes.
Figures and Tables
References
1. Zandberga E, Kozirovskis V, Abols A, Andrejeva D, Purkalne G, Line A. Cell-free microRNAs as diagnostic, prognostic, and predictive biomarkers for lung cancer. Genes Chromosomes Cancer. 2013; 52:356–369.
2. Leal JA, Lleonart ME. MicroRNAs and cancer stem cells: therapeutic approaches and future perspectives. Cancer Lett. 2013; 338:174–183.
3. Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005; 120:635–647.
4. Tufman A, Tian F, Huber RM. Can microRNAs improve the management of lung cancer patients? A clinician's perspective. Theranostics. 2013; 3:953–963.
5. Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004; 64:3753–3756.
6. Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S, et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008; 13:48–57.
7. Franchina T, Amodeo V, Bronte G, Savio G, Ricciardi GR, Picciotto M, et al. Circulating miR-22, miR-24 and miR-34a as novel predictive biomarkers to pemetrexed-based chemotherapy in advanced non-small cell lung cancer. J Cell Physiol. 2014; 229:97–99.
8. Hu Z, Shu Y, Chen Y, Chen J, Dong J, Liu Y, et al. Genetic polymorphisms in the precursor MicroRNA flanking region and non-small cell lung cancer survival. Am J Respir Crit Care Med. 2011; 183:641–648.
9. Wang T, Lv M, Shen S, Zhou S, Wang P, Chen Y, et al. Cell-free microRNA expression profiles in malignant effusion associated with patient survival in non-small cell lung cancer. PLoS One. 2012; 7:e43268.
10. Voortman J, Goto A, Mendiboure J, Sohn JJ, Schetter AJ, Saito M, et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer Res. 2010; 70:8288–8298.
11. Huang W, Hu J, Yang DW, Fan XT, Jin Y, Hou YY, et al. Two microRNA panels to discriminate three subtypes of lung carcinoma in bronchial brushing specimens. Am J Respir Crit Care Med. 2012; 186:1160–1167.
12. Barshack I, Lithwick-Yanai G, Afek A, Rosenblatt K, Tabibian-Keissar H, Zepeniuk M, et al. MicroRNA expression differentiates between primary lung tumors and metastases to the lung. Pathol Res Pract. 2010; 206:578–584.
13. Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009; 106:12085–12090.
14. Abd-El-Fattah AA, Sadik NA, Shaker OG, Aboulftouh ML. Differential microRNAs expression in serum of patients with lung cancer, pulmonary tuberculosis, and pneumonia. Cell Biochem Biophys. 2013; 67:875–884.
15. Shen J, Liu Z, Todd NW, Zhang H, Liao J, Yu L, et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011; 11:374.
16. Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'olio V, Bernard L, et al. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol Med. 2011; 3:495–503.
17. Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014; 32:768–773.
18. Shen J, Liao J, Guarnera MA, Fang H, Cai L, Stass SA, et al. Analysis of MicroRNAs in sputum to improve computed tomography for lung cancer diagnosis. J Thorac Oncol. 2014; 9:33–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download