Abstract
Hypercoagulability disorders are commonly encountered in clinical situations in patients with a variety of cancers. However, several hypercoagulability disorders presenting as first symptoms or signs in cancer patients have rarely been reported. We herein described a case of a woman with adenocarcinoma of the lung presenting with deep vein thrombosis, nonbacterial thrombotic endocarditis, recurrent cerebral embolic infarction, and heart failure.
As a paraneoplastic syndrome, hypercoagulability disorders are known to be commonly associated with a variety of cancer types including lung cancer1,2,3. Approximately 15% of cancer patients suffer from hypercoagulability disorders during their clinical course; 50% of cancer patients show evidence of hypercoagulability disorders on autopsy3,4. Cancer-associated hypercoagulability status may also present as Trousseau's syndrome (a migratory superficial thrombophlebitis), arterial thrombosis, disseminated intravascular coagulation, or nonbacterial thrombotic endocarditis (NBTE, formerly called marantic endocarditis). Venous thromboembolism, such as deep-vein thrombosis (DVT) and pulmonary embolism (PE), is more commonly observed than arterial thrombosis. However, hypercoagulability disorders are a rare presenting symptom or sign of cancer patients, therefore, coexistence of DVT at the time of diagnosis of lung cancer was reported to be 0.1-0.4% in data from California Cancer Registry2.
NBTE, which was introduced in 1936 by Gross and Friedberg5, is characterized by deposition of thrombi on aortic or mitral valves in the absence of bacterial infection6. No definitive clinical features suggest NBTE, in general. However, when a patient shows negative blood culture result and multiple disseminated embolisms, NBTE is more strongly suspected rather than infective endocarditis7,8. Incidence of NBTE is largely not well evaluated; it was 1.25% of cancer patients in an autopsy study7. Systemic embolization to the central nervous system and coronary arteries are often observed in NBTE patients8,9. However, acute heart failure caused by NBTE with cardiac valve lesions is a rare clinical event10.
Association with hypercoagulability disorders is a clinical concern because these cause delay in treatment and worse survival of cancer patients and have a significant adverse effect on quality of life2,11. We herein report on a lung cancer patient who presented with DVT, NBTE, recurrent cerebral embolic infarction, and heart failure.
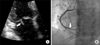
A 61-year-old Korean woman was admitted to the Department of Vascular Surgery, presenting with a tingling sensation on both lower legs and vague chest discomfort for one month. She was a never smoker and had dyspnea (3 on Medical Research Council scale) for two weeks but no fever. She had undergone surgical excision of a benign mass of the left breast and hysterectomy due to uterine prolapse, 15 and five years ago, respectively; denied any postoperative complications and history of renal or hepatic disease. Computed tomogram (CT) angiography of the chest did not show evidence of pulmonary thromboembolism. However, 2×1.2-cm-sized nodular lesion in left lingular division abutting to mediastinal pleura and lymphadenopathies on the left paraaortic and supraclavicular area and both prevascular and subaortic area were noted (Figure 1). Clots in the popliteal and calf vein of the right leg were noted on a CT angiogram for the pelvis and legs (Figure 2). The patient was immediately started on treatment with continuous administration of intravenous heparin. An extensive laboratory work-up was performed in search of underlying hypercoagulability disorders (Table 1). On the fourth day, she was transferred to Department of Center for Lung Cancer for further evaluation of lung cancer. No endobronchial lesion was observed on bronchoscopic examination. Adenocarcinoma was diagnosed pathologically on a biopsy specimen of supraclavicular lymph node. Thyroid transcription factor-1 was positive on immunohistochemistry and activating mutation of epidermal growth factor receptor gene was not demonstrated. On the fifth day, palsy of the lateral gaze of both eyes and double vision was developed suddenly. She underwent brain magnetic resonance angiogram, which demonstrated subacute stage of embolic infarctions on territories of both the middle and posterior cerebral arteries and the right cerebellar hemisphere (Figure 3). Neither stenotic flow nor visible plaque was demonstrated in Doppler ultrasound examination for both carotid arteries. No evidence of distant metastasis was observed on brain magnetic resonance image (MRI), bone scan, and positron emission tomography. Finally, her disease was staged as IIIB; however, she did not want to receive any treatment for her lung cancer. On Transthoracic echocardiogram, vegetation measuring 9 mm in size was observed on the posterior mitral leaflet with moderate mitral regurgitation and the basal inferior wall was akinetic with 50% of left ventricular ejection fraction (Figure 4A). Blood culture of the patient revealed no evidence of bacteremia. Diffuse irregular stenosis (30% of diameter) of the distal portion of the left anterior descending artery and diffuse irregular stenosis (80% of diameter) of the posterolateral branch of the right coronary artery were observed on coronary angiogram (Figure 4B). Intracoronary injection was administered with ReoPro (Abciximab, Eli-Lilly, Indianapolis, IN, USA) and thrombus was aspirated from the right coronary artery. On the eighth day, heparin was switched to warfarin. On the 15th day, follow-up transthoracic echocardiogram showed a decrease in size of the vegetation (4 mm), improvement of mitral regurgitation (mild degree), and normalization of left ventricular ejection fraction. She was discharged on the 17th day. Three weeks later, she visited to the emergency room with sudden development of dysarthria, aphasia, and right hemiplegia. A newly demonstrated hyperacute embolic infarction in the operculum area of the left frontal-parietal junction was observed on her brain MRI. She is receiving rehabilitation treatment for her hemiplegia.
Hypercoagulable state (also called thrombophilia or prothrombotic state) is an abnormal condition that increases the risk of thrombosis. A hypercoagulability of malignancy occurs due to activation of coagulation system by cancer cells. Prothrombotic mechanisms in malignancy associated with procoagulant/fibrinolytic substances secreted by cancer cells, tumor cell-host cell interactions and other nonspecific factors such as necrosis or hemodynamic compromise. Venous thromboembolism is more common in mucin-producing adenocarcinoma of pancreas and gastrointestinal tract, lung cancer, ovarian cancer12.
After presence of a hypercoagulable state in stomach cancer patients was found by Trousseau in 1865, venous thrombosis has been a common clinical feature in patients with a variety of cancer types, including lung cancer13. Association of venous thrombosis, such as DVT and PE, in cancer patients has been well established and can have an adverse effect on survival11,14. However, in general, NBTE and its embolization to coronary and/or cerebral arteries have not been well evaluated; most were reported after the diagnosis of cancer7,10. With our limited knowledge, this case shows a unique clinical presentation of DVT, NBTE, recurrent cerebral embolic infarction, and heart failure at the time of diagnosis of lung cancer. In addition, involvement of major organs, including brain and heart at presentation may cause a delay in diagnosis of cancer because patients could initially be treated by a cardiologist or neurologist. Their coexistence can have a significant impact on quality of life of cancer patients and cause a delay in treatment or administration of less effective treatment that causes worse survival.
Therefore, clinicians need to pay attention to the possibility of coexistence with severe forms of hypercoagulability disorders at the time of diagnosis. Early identification and aggressive intervention could be essential in preventing delay of diagnosis or treatment.
Figures and Tables
Figure 1
A 2×1.2-cm-sized nodular lesion in the left lingular division abutting to mediastinal pleura (A, arrow) and lymphadenopathies on the left paraaortic area, on chest CT scan at the time of diagnosis (B, arrow).

Figure 2
Computed tomogram angiogram for the legs showing venous thrombosis at the popliteal vein (A, arrow) and calf vein (B, arrow) of the right leg.
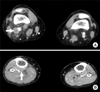
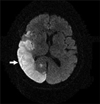
Figure 3
Brain magnetic resonance image showing a subacute stage infarction involving a territory of the right middle cerebral artery.
References
1. Falanga A, Ofosu FA, Delaini F, Oldani E, Dewar L, Lui L, et al. The hypercoagulable state in cancer patients: evidence for impaired thrombin inhibitions. Blood Coagul Fibrinolysis. 1994; 5:Suppl 1. S19–S23.
2. Dipasco PJ, Misra S, Koniaris LG, Moffat FL Jr. Thrombophilic state in cancer, part I: biology, incidence, and risk factors. J Surg Oncol. 2011; 104:316–322.
3. Glassman AB. Hemostatic abnormalities associated with cancer and its therapy. Ann Clin Lab Sci. 1997; 27:391–395.
4. Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost. 1999; 25:173–182.
5. Gross L, Friedberg CK. Nonbacterial thrombotic endocarditis: classification and general description. Arch Intern Med. 1936; 58:620–640.
6. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007; 12:518–523.
7. Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987; 113:773–784.
8. Borowski A, Ghodsizad A, Cohnen M, Gams E. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg. 2005; 79:2145–2147.
9. Chino F, Kodama A, Otake M, Dock DS. Nonbacterial thrombotic endocarditis in a Japanese autopsy sample: a review of eighty cases. Am Heart J. 1975; 90:190–198.
10. Taniyama D, Yamamoto R, Kawasaki M, Kamata H, Miyamoto K, Mashimo S, et al. Nonbacterial thrombotic endocarditis leading to acute heart failure due to aortic stenosis in a patient with lung cancer. Intern Med. 2013; 52:1617–1620.
11. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000; 343:1846–1850.
12. Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002; 4:465–473.
13. Connolly GC, Dalal M, Lin J, Khorana AA. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer. 2012; 78:253–258.
14. Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. Thrombosis and cancer. Nat Rev Clin Oncol. 2012; 9:437–449.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download