Abstract
We report a case of invasive pulmonary aspergillosis invading the mediastinum and the left atrium. A 70-year-old woman was hospitalized for dyspnea. She had been well controlled for her diabetes mellitus and hypertension. The chest X-ray disclosed mediastinal widening, and the computed tomography scan of the chest showed that there was a large mediastinal mass and this lesion extended into the left atrium and right bronchus. The cardiac echocardiography showed that a huge mediastinal cystic mass compressed in the right atrium and a hyperechoic polypoid lesion in the left. The pathology from the bronchoscopic biopsy observed abundant fungal hyphae which was stained with periodic acid-Schiff and Gomori's methenamine silver. Despite the treatment with antifungal agents, she died from cardiac tamponade after three months. Invasive pulmonary aspergillosis, which involves the mediastinum and the heart, is very rare in immunocompetent patients.
Although invasive pulmonary aspergillosis is not rare in immunocompromised patients, it is very rare in immunocompetent patients1. In addition, invasive aspergillosis, which involves mediastinum in an immunocompetent host, was reported only once in the literature2. To the best of our knowledge, this is the second case report of invasive pulmonary aspergillosis extending to the mediastium and the left atrium in an immunocompetent patient.
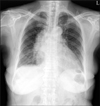
A 70-year-old woman visited our hospital for mild dyspnea, cough, and sputum on March 6, 2012. She had been in a well-controlled state of her diabetes and hypertension since 2000. The level of glycosylated hemoglobin A1c was 6.1% on admission. She had no other medical history and did not administer medications such as corticosteroid or antibiotics. On admission, body temperature was 37.4℃, blood pressure 100/70 mm Hg, and pulse rate 92 beats per minute. Breath sounds were diminished over the right lung. The laboratory test showed a white blood cell count of 7,760/µL and a C-reactive protein of 0.39 mg/dL. A plain chest radiograph showed prominent mass opacity in the right hilum (Figure 1). A computed tomography (CT) scan of the chest revealed an ill-defined poorly enhanced mediastinal mass invading the subcarina, the right hilum, and the left atrium, a protruding lesion in the right bronchus intermedius, peribronchovascular interstitial thickening around the right bronchus, and a small amount of pleural effusion in the right hemithorax. The mass in the mediastinum compressed the right atrium and the right ventricle (Figure 2). The diagnostic thoracentesis revealed a lymphocyte dominant exudate, a white blood cell count of 1,630/µL (lymphocyte 43%), albumin of 2.2 g/dL, lactate dehydrogenase of 883 U/L, glucose of 210 mg/dL, and adenosine deaminase of 15I U/L. The cytologic examination observed no malignant cells in the pleural effusion. A CT-guided percutaneous needle aspiration biopsy (PCNA) of the mediastinal mass was conducted. The pathology revealed necrotic tissues only with no evidence of malignancy. In the fiberoptic bronchoscopy, the orifice of the right bronchus intermedius was nearly totally obstructed by a bloody mass (Figure 3). The pathology from the bronchoscopic biopsy observed abundant fungal hyphae. The result of stains with periodic acid-Schiff and Gomori's methenamine silver for fungi was positive for fungal hyphae, and the acid-fasting staining showed a negative result for acid-fast bacilli (Figure 4). The serologic test for Aspergillus fumigatus was negative. An echocardiography showed an ejection fraction of 63% and normal global left ventricular systolic function with no regional wall motion abnormality. However, there was a huge mediastinal mass compressing the heart and a hyperechoic polypoid lesion in the left atrium (Figure 5). She was diagnosed with invasive pulmonary aspergillosis involving the heart and thus, amphotericin B (0.25 mg/kg/day) was prescribed intravenously.
Fourteen days after hospitalization, dyspnea got more worsened. A follow-up chest radiograph showed aggravated pleural effusion in the right hemithorax and worsened cardiomegaly. The effusion was surgically drained by video-assisted thoracic surgical drainage. The antifungal agent was voriconazole, initially, 6 mg/kg intravenously every 12 hours, and then changed to 4 mg/kg intravenously every 12 hours.
Two weeks later, the chest tube was removed, but found a cardiac tamponade. This was why the sono-guided pig-tail catheter was inserted into the pericardium. Seven days later, the catheter was removed and the endobronchial aspergilloma was removed using fiberoptic bronchoscope for palliation. Two months later, a small amount of pleural effusion still remained. The mediastinal mass decreased and her dyspnea improved. She was discharged from a hospital.
One week later, her dyspnea got worsened. The laboratory test showed thrombocytopenia of the platelet cell count of 26,000/µL. The body temperature was 36.5℃, blood pressure 90/60 mm Hg, and pulse rate 130 beats per minute. A CT scan showed that the amount of pleural effusion and pericardial fluid increased more. The mediastinal mass increased in size and still compressed the heart, and the heart was deviated to the left side (Figure 6). The echocardiography observed increased polypoid lesion in the left atrium, and the lesion shifted to mitral valve during the diastolic phase (Figure 7). A pericardiocentesis with drainage was performed to relieve cardiac tamponade. Despite the medical treatment, she wasn't improved. When the patient and her family refused to receive any more intensive cares, she died seven days later. An autopsy was also refused.
Aspergillus fumigatus is a common pathogen in human airways and causes various diseases ranging from mild hypersensitivity reaction to more severe invasive diseases. The major form of pulmonary aspergillosis ranges from benign aspergilloma to invasive aspergillosis, which is rapidly progressive, and its mortality rate is as high as at 50%, despite the treatment with proper antifungal agents including amphotericin B and voriconazole3,4. In this case, her disease progressed rapidly and the antifungal treatment was not effective any longer. The time to take from the initial manifestation to her death was only three months.
Invasive pulmonary aspergillosis in an immunocompetent host is very rare. In this case, she had been treated for diabetes mellitus, but she was in a well-controlled state. There was no past medical history of immunosuppression or underlying malignancy. There was no leukocytopenia in the laboratory findings, and the test for human immunodeficiency virus was negative. This is why we concluded that she was an immunocompetent host.
On admission, we suspected that the right hilar mass would be a lung cancer. However, the specimens obtained from PCNA showed necrotic tissues only and no evidence of malignancy. The pathology from the bronchoscopic biopsy showed abundant fungal hyphae. Because the endobrochial mass was connected to the mediastium in chest CT, we diagnosed this mediastinal mass as invasive aspergillosis. But there was a possibility of contamination by fungi in the air on bronchoscopic biopsy.
Initially, we thought that the intracardiac lesion would be thrombus with invasive aspergillosis. But there was no response of anticoagulation, and the CT scan revealed that the mediastinal mass was directly bridged to the endobroncheal lesion and the left atrial intracardiac mass. So, we thought that the endocardium in the left atrium was also directly invaded by the Aspergillus. However, in our case, we had only an imaging evidence of cardiac involvement of aspergillosis and no histological evidence, for her family refused to do autopsy. This was the limitation for this case.
In our case, the pathology from the bronchoscopic biopsy showed invasive aspergillosis. But the result from galactomannan assay (GMA) was negative. The galactomannan, a principal ingredient of Aspergillus hypae cell membrane, was released into the blood when Aspergillus invaded the blood vessel5. So, the GMA using enzyme immunoassay was used to diagnose Aspergillus infection. The galactomanan assay has a low sensitivity and high specificity. In one study, sensitivity, specificity, positive predictive value, or negative predictive value of the GMA was at 50% (95% confidence interval [CI], 26-74%), 88% (95% CI, 81-93%), 38% (95% CI, 19-59%), or 93% (95% CI, 86-97%), respectively6. The GMA was also affected by the activity of Aspergillus invasion. The sensitivity of GMA is very low in immunocompetent patients or in non-invasive Aspergillus infection. However, the sensitivity was high in immunocompromised patients or in the fast progression of disease7,8,9. When the host immunity is normal and the aspergillus invasion of blood vessel is less than the standard, the result from GMA can be normal in invasive aspergillosis patients. In our case, although aspergillus invaded the blood vessel and the disease was progressive fast, we thought that the negative result of the GMA resulted from the normal host immunity and low sensitivity of the GMA.
Invasive pulmonary aspergillosis is responsible for a high mortality rate ranging from 30% to 50%, despite the proper treatment3,4. And the prognosis of aspergillosis with cardiac involvement is very poor, usually due to the delayed diagnosis10,11. As we think that the causes of death are heart failure and cardiac tamponade, the progression of disease from admission to death is very rapid and aggressive. To improve the prognosis of invasive aspergillosis, it is important to recognize the clinical features of extra-pulmonary aspergillosis including cardiac invasion and to institute the aggressive anti-fungal treatment12.
In conclusion, Invasive pulmonary aspergillosis with cardiac involvement occurs even in immunocompetent patients. The prognosis of aspergillosis with cardiac invasion is very poor, even though it is occurred in immunocompetent patients. Thus, it is important to recognize the clinical features of extra-pulmonary aspergillosis as quickly as possible and to institute the aggressive proper treatment as early as possible. New approaches and new therapies would be also needed to improve the outcome of invasive aspergillosis in the patient groups of high mortality.
Figures and Tables
Figure 2
The chest computed tomography scan shows the mediastinal mass compressing the right atrium and the right ventricle (arrowheads). The mediastinal mass is connected to the left atrium, the right hilum, and subcarina (arrows).
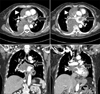
Figure 3
In bronchofiberscopic examination, the orifice of right bronchus intermedius is nearly completely obstructed by a bloody mass.

Figure 4
(A) The bronchoscopic biopsy of the mass observed in abundant fungal hyphae (H&E stain, ×20). (B) Fungal hyphae were positive stained with periodic acid-Schiff stain (×40).

Figure 5
The polypoid mass in the left atrium was shown (arrows) in parasternal long axis view (A) and parasternal short axis view (B) of echocardiogram. The mediastinal mass (arrowheads) compressed the right atrium and the right ventricle in subcostal view (C, D) of echocardiogram. Ao: aorta; AV: aortic valve; LA: left atrium; LV: left ventricle; MV: mitral valve; PA: pulmonary artery; RA: right atrium; RV: right ventricle.
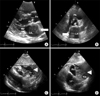
Figure 6
The chest computed tomography scan observed increased right hilar and subcarinal mass (arrows) compressing the superior vena cava, increased right mediastinal mass compressing the right atrium and right ventricle (arrowheads), and the heart deviated to the left.
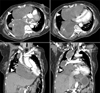
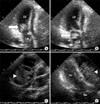
Figure 7
The polypoid mass in the left atrium is shown (arrow). The mass shifted to the mitral valve during the diastolic phase in two apical chamber views (A: diastolic phase, B: systolic phase) of echocardiogram. The mediastinal mass (arrowheads) increased more and compressed the right atrium and the right ventricle in the parasternal short asix view (C) and the two apical chamber views (D) of echocardiogram. LA: left atrium; LV: left ventricle; MV: mitral valve; RA: right atrium; RV: right ventricle.
References
1. Allam MF, Del Castillo AS, Diaz-Molina C, Navajas RF. Invasive pulmonary aspergillosis: identification of risk factors. Scand J Infect Dis. 2002; 34:819–822.
2. Shakoor MT, Ayub S, Ayub Z, Mahmood F. Fulminant invasive aspergillosis of the mediastinum in an immunocompetent host: a case report. J Med Case Rep. 2012; 6:311.
3. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347:408–415.
4. Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. I3 Aspergillus Study Group. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore). 2000; 79:250–260.
5. Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004; 4:349–357.
6. Song KH, Lee S, Jang HC, Jeon JH, Park WB, Park KU, et al. Diagnostic usefulness of galactomannan assay for invasive aspergillosis. Infect Chemother. 2009; 41:82–89.
7. Aquino VR, Goldani LZ, Pasqualotto AC. Update on the contribution of galactomannan for the diagnosis of invasive aspergillosis. Mycopathologia. 2007; 163:191–202.
8. Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005; 73:494–503.
9. Pasqualotto AC, Denning DW. Post-operative aspergillosis. Clin Microbiol Infect. 2006; 12:1060–1076.
10. Woods GL, Wood RP, Shaw BW Jr. Aspergillus endocarditis in patients without prior cardiovascular surgery: report of a case in a liver transplant recipient and review. Rev Infect Dis. 1989; 11:263–272.
11. Mullen P, Jude C, Borkon M, Porterfield J, Walsh TJ. Aspergillus mural endocarditis. Clinical and echocardiographic diagnosis. Chest. 1986; 90:451–452.
12. Hori A, Kami M, Kishi Y, Machida U, Matsumura T, Kashima T. Clinical significance of extra-pulmonary involvement of invasive aspergillosis: a retrospective autopsy-based study of 107 patients. J Hosp Infect. 2002; 50:175–182.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download