Abstract
Recently, the incidence of pulmonary cryptococcosis is gradually increasing in rheumatoid arthritis (RA) patients. Pulmonary rheumatoid nodules (PRN) are rare manifestations of RA. Eighteen months ago, a 65-year old woman was admitted to hospital due to multiple nodules (2.5×2.1×2 cm) with cavitations in the right lower lobe. She was diagnosed with RA three year ago. She had been taking methotrexate, leflunomide, and triamcinolone. A video-assisted thoracoscopic surgery biopsy was performed and PRN was diagnosed. However, a newly growing huge opacity with cavitation was detected in the same site. Pulmonary cryptococcal infection was diagnosed through a transthoracic computed tomograpy guided needle biopsy. Cryptococcus antigen was detected in serum but not in cerebrospinal fluid. The patient was treated with oral fluconazole which resulted clinical improvement and regression of the nodule on a series of radiography. Herein, we report the case of pulmonary cryptococcosis occurring in the same location as that of the PRN.
The prevalence rate of pulmonary rheumatoid nodules ranges from <1% on chest radiography (CXR) to 32% in lung biopsies of rheumatic arthritis (RA) patients1,2. Pulmonary cryptococcosis may affect immunocompetent hosts but is more common in immunocompromised hosts, especially, in patients with human immunodeficiency virus infection, diabetes, malignancy, and organ transplantation. It can also develop as a complication of RA therapy that includes tumour necrosis factor alpha inhibitor or disease-modifying anti-rheumatic drug use3. However, clinical and radiographic features are difficult to distinguish between these two disease conditions. Pulmonary rheumatoid nodules usually do not need treatment, but disseminated cryptococcosis needs urgent antifungal therapy. Cryptococcosis is more often reported in RA patients, but pulmonary cryptococcosis that mimics a rheumatoid nodule in the same lesion has not been reported before. We report a case of pulmonary cryptococcosis that presented as a cavitary lung mass in the same site as that of the previous rheumatoid nodule.
Our patient was a 65-year-old Korean woman with 3-year history of RA, which had been treated with methotrexate (10 mg/wk), leflunomide (20 mg/day), and triamcinolone (0.5 mg/day). She had a 2-year history of known diabetes, which had been well controlled with metformin (500 mg) once daily. Additionally she had been taking anti-hypertensive drugs, (amlodipine 5 mg and losartan 50 mg) once daily for 5 years. She had no history of smoking and alcohol abuse. She had a mild cough, weight loss of 5 kg during the last 6 months (body mass index [BMI], 21.6 kg/m2). At her first visit to our clinic, she did not look very ill; her vital signs were stable. She had four rheumatoid nodules in both hands. Auscultation of the right lower lung field revealed crackle. Laboratory test results showed the following: white blood cell (WBC), 5,800/µL; red blood cell (RBC), 10.5 g/dL; haematocrit (Hct), 29.6%; platelet, 256,000/µL; segmented neutrophil, 71.4%; lymphocyte, 16%; monocyte, 10.7%; eosinophil, 1.3%; and basophil, 0.6%; high-sensitivity C-reactive protein, 2.1 mg/L; erythrocyte sedimentation rate (ESR), 21 mm/hr; rheumatoid factor (RF), <20 IU/mL; and haemoglobin A1c, 5.5%. Serum electrolyte, liver, and renal function test results were normal; serum Aspergillus antigen, sputum acid-fast bacilli (AFB) smear and culture test results were negative. The CXR and chest computed tomography (CT) scans demonstrated irregularly shaped two cavitary nodules sized 2.5×2.3×2 cm and 1×1×0.8 cm, respectively, in the right lower lung lobe (Figure 1A). The lesion continued to gradually grow in size during the last 6 months before admission. Diagnostic bronchoscopy revealed no endobronchial lesion; the bronchial washing specimen from the right lower lobe showed no evidence of pulmonary tuberculosis or malignancy. The mass was surgically resected by wedge resection via video-associated thoracoscopy because the tissue from the initial percutaneous needle biopsy was non-diagnostic, and malignancy or other infectious diseases were suspected and had to be ruled out. Surgical biopsy confirmed the rheumatoid nodule from an area of necrotising granulomatous inflammation, with a negative AFB smear or culture result (Figure 2). The RA therapy was maintained with the same regimen, and she was clinically stable for 18 months. However, she presented with general weakness, anorexia, and an additional weight loss of 3 kg in the past 3 months (BMI, 19.34 kg/m2). She took herb medication for diabetes control during the 3-month period. However, newly growing huge opacity with cavitation in the same location and additional multiple nodules were detected on CXR (Figure 1C). Laboratory tests revealed the following: WBC, 11,800/µL; RBC, 10.2 g/dL; Hct, 31%; platelet, 466,000/µL; segmented neutrophil, 81%; lymphocyte, 10%; monocyte, 3%; eosinophil, 3%; basophil, 1%; high-sensitivity C-reactive protein, 18.9 mg/L; ESR, 120 mm/hr; RF, 34.5 IU/mL; and haemoglobin A1c, 5.0%. Bronchoscopic findings showed a negative Aspergillus antigen titre and negative AFB smear and culture results. CT-guided transthoracic needle biopsy was performed to determine other causes of infection or malignancy. Pathologic findings were compatible with pulmonary cryptococcosis (Figure 2). Cryptococcus antigen was found in serum but not in cerebrospinal fluid. The Cryptococcus antigen titre could not be measured. Cerebrospinal fluid analysis result was within the reference range. She was treated with oral fluconazole (400 mg) once daily. Four months later, she became well without any symptoms. Follow-up imaging studies showed improvement of the previously noted lesion (Figure 1D).
Pulmonary rheumatoid nodule is a specific manifestation of RA; it occurs more frequently in males, smokers and patients with positive RF test results or subcutaneous rheumatoid nodules1. Most RA patients with pulmonary rheumatoid nodule do not require a specific treatment or experience any cause for a clinical episode. Up to 50% of pulmonary rheumatoid nodules may cavitate and be accompanied by an associated pleural effusion, pneumothorax, or hydropneumothorax. Radiologic diagnosis is performed for cases with multiple, round, well-defined rheumatoid nodules of similar sizes and subpleural location2.
Cryptococcosis is an invasive fungal infection caused predominantly by Cryptococcus neoformans or Cryptococcus gattii4. Pulmonary cryptococcosis is caused by the inhalation of cryptococcal particles into the lungs and granuloma or pneumonia development. Although immunocompromised hosts are considered to be at greatest risk for symptomatic and life-threatening pulmonary cryptococcal infections, severe complications also may occur in healthy hosts5. Most pulmonary cryptococcosis cases in immunocompetent patients are clinically silent and do not require therapy. Cryptococcosis is more often reported in RA patients, and the infection risk may increase owing to the disease itself, along with intrinsic cellular immunity alterations or complications from drugs used to control RA6,7,8. However, clinical and radiologic characteristics of pulmonary cryptococcosis in RA patients have not yet been fully described. Multiple nodules of 10-30 mm in diameter, consolidation, ground-glass attenuation, and cavitary lesions were the main radiologic features in RA patients9. Unfortunately, because these radiologic findings are similar to those of pulmonary rheumatoid nodule, distinguishing pulmonary cryptococcosis from pulmonary rheumatoid nodules is difficult10,11. Furthermore, because most clinical findings are non specific, differentiating pulmonary cryptococcosis from rheumatoid nodules based on symptomatic episodes is also difficult. Moreover, laboratory findings such as C-reactive protein level, RF level, and ESR level were not typical in this patient. One report described clinical and radiologic differences in the features of pulmonary cryptococcosis between immunocompromised and immunocompetent patients. The prevalence of symptoms was similar between the two groups. Half of the RA patients with pulmonary cryptococcosis did not have any clinical episode; the remaining patients had only flu-like symptoms9.
In our patient, pulmonary cryptococcosis unlikely developed in the site of the previous rheumatoid nodule. Although she lost weight owing to the herb medication, RA disease activity did not change significantly and the infectious sign was not clear. Without tissue biopsy, the nodules were difficult for us to diagnose as pulmonary cryptococcosis. To our knowledge, the presence of cryptococcosis in a pulmonary rheumatoid nodule is extremely rare and has not been previously reported12,13,14. Pulmonary rheumatoid nodules are composed of fibrinoid necrosis surrounded by epithelial cells and macrophages with secondary inflammation and are usually localised in the interlobular septa or pleura and are normally asymptomatic15. However, pulmonary rheumatoid nodules may occasionally undergo infection and cavitation. In fact, fungal infection tends to be associated with pre-existing pulmonary rheumatoid nodules with cavities such as tuberculosis, bronchiectasis, cavitating carcinoma or amyloidosis13,14,15. Moreover, RA patients receiving long-standing immunosuppressive agents who have increased incidence of pulmonary fibrosis have a higher risk of for pulmonary fungal infection. In conclusion, rheumatoid nodules might be a possible predisposing cause of pulmonary cryptococcosis in RA patients receivng immunosuppressive drugs, thus warranting clinical suspicion and early intensive diagnostic approach.
Figures and Tables
Figure 1
Radiologic findings. (A) Multifocal area of small nodules with cavitation in the right lower lung fields at 21 months prior to admission. (B) Postoperative sequalae was noted after 11 months of video-assisted thoracoscopic surgery biopsy. (C) Huge opacity with cavitation growing in the right lower lung fields at the time of admission. (D) Chest posteroanterior view and computed tomographic image showing a decrease in the size of the consolidative huge opacity after treatment with oral fluconazole at 400 mg daily for 4 months.
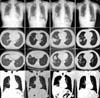
Figure 2
Histological features of the first (A and B) and newly developed pulmonary lesions (C-E). (A) Low magnification of the first lesion located in the subpleural lung region showing necrotising granulomas. (B) The granulomas consisting of central necrosis (left upper side) surrounded by palisading epithelioid histiocytes, multinucleated giant cells, and chronic inflammation (right lower side). No definite histological features suggestive of vasculitides were observed. Special stainings including Ziehl-Neelsen, Grocott-Gomori methenamine silver, and periodic acid-Schiff staining showed no acid-fast bacilli or fungi (figures not shown). These features combined with the clinical findings suggest a diagnosis of necrobiotic nodules caused by rheumatoid arthritis. (C) The newly developed lesion showing granulomas containing epithelioid histiocytes, multinucleated giant cells, and chronic inflammation. Some of the epithelioid histiocytes had bubbly cytoplasms. The lesion was accompanied by a necrotic fragment (inset). (D) At higher magnification, the typical round, pale intracellular organisms with a clear halo could be seen in the viable epithelioid histiocytes. (E) The periodic acid-Schiff stain is positive for round yeasts. These features are compatible with a diagnosis of cryptococcosis (A, H&E stain, ×12.5; B, H&E stain, ×100; C, H&E stain, ×200; D, H&E stain, ×400; E, periodic acid-Schiff stain, ×400).

References
1. Anaya JM, Diethelm L, Ortiz LA, Gutierrez M, Citera G, Welsh RA, et al. Pulmonary involvement in rheumatoid arthritis. Semin Arthritis Rheum. 1995; 24:242–254.
2. Gomez Herrero H, Arraiza Sarasa M, Rubio Marco I, Garcia de Eulate Martin-Moro I. Pulmonary rheumatoid nodules: presentation, methods, diagnosis and progression in reference to 5 cases. Reumatol Clin. 2012; 8:212–215.
3. Aberg JA, Mundy LM, Powderly WG. Pulmonary cryptococcosis in patients without HIV infection. Chest. 1999; 115:734–740.
4. Kim YS, Lee IH, Kim HS, Jin SS, Lee JH, Kim SK, et al. Pulmonary cryptococcosis mimicking primary lung cancer with multiple lung metastases. Tuberc Respir Dis. 2012; 73:182–186.
5. Nunez M, Peacock JE Jr, Chin R Jr. Pulmonary cryptococcosis in the immunocompetent host. Therapy with oral fluconazole: a report of four cases and a review of the literature. Chest. 2000; 118:527–534.
6. Caporali R, Caprioli M, Bobbio-Pallavicini F, Montecucco C. DMARDS and infections in rheumatoid arthritis. Autoimmun Rev. 2008; 8:139–143.
7. Hage CA, Wood KL, Winer-Muram HT, Wilson SJ, Sarosi G, Knox KS. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003; 124:2395–2397.
8. Yoo HG, Yu HM, Jun JB, Jeon HS, Yoo WH. Risk factors of severe infections in patients with rheumatoid arthritis treated with leflunomide. Mod Rheumatol. 2013; 23:709–715.
9. Yanagawa N, Sakai F, Takemura T, Ishikawa S, Takaki Y, Hishima T, et al. Pulmonary cryptococcosis in rheumatoid arthritis (RA) patients: comparison of imaging characteristics among RA, acquired immunodeficiency syndrome, and immunocompetent patients. Eur J Radiol. 2013; 82:2035–2042.
10. Morita Y, Katoh S, Watanabe H, Harada H, Uno E, Satoh M, et al. Rheumatoid nodules of the lung in a patient with palindromic rheumatism. Intern Med. 1992; 31:951–954.
11. Song KD, Lee KS, Chung MP, Kwon OJ, Kim TS, Yi CA, et al. Pulmonary cryptococcosis: imaging findings in 23 non-AIDS patients. Korean J Radiol. 2010; 11:407–416.
12. Adelman HM, Dupont EL, Flannery MT, Wallach PM. Case report: recurrent pneumothorax in a patient with rheumatoid arthritis. Am J Med Sci. 1994; 308:171–172.
13. Cavazza A, Paci M, Turrini E, Dallari R, Rossi G. Fungus colonisation of pulmonary rheumatoid nodule. J Clin Pathol. 2003; 56:636–637.
14. Winne L, Praet M, Brusselle G, Veys E, Mielants H. Bilateral spontaneous pneumothorax in a patient with pulmonary rheumatoid nodules, secondary infected by Aspergillus. Clin Rheumatol. 2007; 26:1180–1182.
15. Scully RE, Mark EJ, McNeely WF, Ebeling SH, Phillips LD. Case records of the Massachusetts General Hospital Weekly clinicopathological exercises. Case 20-1997. A 74-year-old man with progressive cough, dyspnea, and pleural thickening. N Engl J Med. 1997; 336:1895–1903.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download