Abstract
The combination chemotherapy of irinotecan with 5-fluorouracil and leucovorin (FOLFIRI regimen) was recently proven to be beneficial in patients with advanced colorectal cancer. Pulmonary toxicity is very rare in adverse effects of irinotecan. No case of organizing pneumonia (also known as bronchiolitis obliterans organizing pneumonia) associated with FOLFIRI chemotherapy has been reported. We experienced a case of a 62-year-old man who presented persistent dry cough and progressive dyspnea after receiving chemotherapy with FOLFIRI regimen. After surgical lung biopsy, the patient was diagnosed with FOLFIRI chemotherapy-induced organizing pneumonia which was successfully treated with steroid therapy.
Colorectal cancer is ranked as the fourth leading cause of cancer-related death worldwide1. Recent advances in chemotherapy lead to the improvement in survival of patients with advanced colorectal cancer. In the past, 5-fluorouracil (5-FU) single therapy was used for advanced colorectal cancer. However, an introduction of newer cytotoxic agents, irinotecan and oxaliplatin, has changed first-line chemotherapy regimen for advanced colorectal cancer2,3. Response rate has been increased up to 53% by combining irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) to 5-FU and leucovorin for advanced colorectal cancer4.
Oxaliplatin and irinotecan have some adverse effects including hematologic, gastrointestinal, and neurologic problems5,6. However, pulmonary toxicity associated with these agents is rare. Particularly, interstitial lung disease (ILD) associated with FOLFIRI chemotherapy has hardly been reported.
We experienced a patient with symptoms and signs of ILD during FOLFIRI chemotherapy, more specifically organizing pneumonia confirmed by surgical lung biopsy and would like to report this case with a review of the literature.
A 62-year-old man was admitted to our hospital with fever, dry cough, and dyspnea, developed 10 days after 11th FOLFIRI chemotherapy. He was diagnosed with advanced colorectal cancer one year ago and underwent abdominoperineal resection. Three months after surgery, FOLFOX (oxaliplatin 85 mg/m2 for 2 hours on day 1; leucovorin 200 mg/m2 for 2 hours added on days 1 and 2; 5-FU 400 mg/m2 as an intravenous bolus injection and 600 mg/m2 in continuous infusion for 22 hours added on days 1 and 2) chemotherapy was done. Lung metastasis was found after three cycles of FOLFOX and the regimen of chemotherapy was changed to FOLFIRI (irinotecan 180 mg/m2 for 2 hours on day 1; leucovorin 200 mg/m2 for 2 hours added on days 1 and 2; 5-FU 400 mg/m2 as an intravenous bolus injection and 600 mg/m2 in continuous infusion for 22 hours added on days 1 and 2). Thereafter he was administered for 11 cycles of FOLFIRI regimen every two weeks. He was an ex-smoker with 50 pack-years and had no history of any other pulmonary disease except lung metastasis.
His vital signs on admission were followings: blood pressure of 147/96 mm Hg, pulse rate of 99/min, respiratory rate of 22/min, body temperate of 38.4℃, and oxygen saturation of 95%. Auscultation of lungs revealed coarse breathing sound with crackle on right upper and lower lung fields. Laboratory findings were as follows: white blood cell 16,750/mm3 (neutrophil 89%), hemoglobin 9.8 g/dL, platelet 252,000/mm3, C-reactive protein 12.96 mg/L, and erythrocyte sedimentation rate 61 mm/hr. Chest X-ray on admission showed multiple patchy consolidation on right upper, right lower and left lower lung fields. Chest high resolution computed tomography (HRCT) revealed multifocal patchy ground glass opacities and consolidation on both lungs, mainly right lung (Figure 1).
Initially, we suspected bacterial pneumonia. Levofloxacin and piperacillin-tazobactam were started intravenously. Blood and sputum cultures were sterile. Bronchoalveolar lavage (BAL) was performed. BAL fluid revealed white blood cell of 1,520/µL (neutrophil 23%, lymphocyte 29%, histiocyte 34%, and eosinophil 12%). On BAL fluid test, cultures for bacteria and fungus, and acid-fast bacilli smear were negative. Polymerase chain reaction for Mycobacterium tuberculosis, Pneumocystis jirovecii, cytomegalovirus, and other common respiratory virus were all negative. On admission day 12, the patient complained of persistent dry cough and aggravated dyspnea. However, he did not have any other extrapulmonary symptoms including arthralgia and skin rash. Autoimmune examination was done and antinuclear antibody (Ab), antineutrophil cytoplasmic Ab, anti-Scl-70 Ab, anti Jo-1 Ab, anti SS-A/B Ab were all negative and rheumatoid factor was 11 IU/mL (normal range, 3-18 IU/mL). Chest HRCT showed aggravated multiple patchy consolidation on both lungs (Figure 2A). On day 14, video-assisted thoracoscopic (VATS) lung biopsy was performed and the pathology showed organizing pneumonia (Figure 3). Treatment with intravenous methylprednisolone 60 mg/day was started. His cough and dyspnea were much improved four days after steroid treatment. He was discharged on day 30. Steroid was gradually tapered and discontinued in the subsequent two months. Chest HRCT, taken two months after discharge showed complete resolution of lung lesion (Figure 2B).
Recent studies have recommended oxaliplatin (FOLFOX)-or irinotecan (FOLFIRI)-based regimens as first-line chemotherapy for advanced colorectal cancer2,3. Although ILD associated with cancer chemotherapeutic agent is a serious complication, potentially lead to death, there are few reports about ILD associated with oxaliplatin or irinotecan.
This is a case of organizing pneumonia developed after receiving chemotherapy with FOLFOX and FOLFIRI regimen. Initially, we suspected infectious pneumonia and administered empirical antibiotics. However, there was no clinical or radiological improvement. On investigational tests taken with blood, sputum and BAL sample, no evidence of causative pathogen was found. VATS lung biopsy finding was compatible with organizing pneumonia. We considered the possibility of drug induced organizing pneumonia after excluding rheumatologic disease. According to Shimura et al.7, the median duration from last FOLFOX to an ILD-related episode was 20 days (range, 0-179 days). In this case, oxaliplatin was less likely as the drug causing ILD because symptoms and signs of ILD developed eight months after completion of last FOLFOX. Other components of FOLFOX and FOLFIRI regimen, 5-FU and leucovorin have been used clinically for the past decades without adverse effect of ILD. Therefore, we assessed that irinotecan was most likely the drug inducing organizing pneumonia in this case. However we could not completely exclude the possibility of ILD developed by drug interaction between irinotecan and 5-FU.
The phase II trial of irinotecan in patients with metastatic colorectal cancer showed the incidence rate of ILD to be 0.8%8. Yoshii et al.9 stated in postmarketing surveillance data on 8,864 participants that three of 18 patients diagnosed with ILD by computed tomography scan showed to have organizing pneumonia pattern. All of them had good response with steroid therapy. Another recent retrospective study, focused on 734 patients with colorectal cancer treated using FOLFOX or FOLFIRI, described that 11 patients (1.5%) were clinically diagnosed with ILD7. Among them, 10 patients received steroid therapy but the four died. These results indicate that ILD constitutes a critical complication in FOLFOX or FOLFIRI chemotherapy for colorectal cancer. It is comparable with reports on gefitinib, demonstrating that it can cause ILD at about 1%-5% in non-small-cell lung cancer with 30% of ILD-related mortality 10,11.
FOLFIRI chemotherapy induced ILD has been very rarely reported and there has been only one histologically confirmed report on irinotecan induced interstitial pneumonitis12. This is the first case report on histologically proven FOLFIRI chemotherapy associated organizing pneumonia in Korea, in which irinotecan was considered to be the most causable drug inducing organizing pneumonia. It is not known about the mechanism how irinotecan leads to ILD. And the relationship between disease onset and chemotherapy duration, response rate of steroid therapy and prognosis are not apparent until now. With regard to the risk factors for ILD associated with FOLFIRI chemotherapy, most patients in previous reports had history of asthma, heavy smoking or lung resection12,13. The patient in this case had 50 pack-years smoking history and lung metastasis before he was diagnosed with organizing pneumonia. Therefore, we infer that FOLFIRI chemotherapy could be more hazardous to patients with preexisting lung disease.
In conclusion, this is the first case report on histologically proven FOLFIRI chemotherapy associated organizing pneumonia. Clinicians using FOLFIRI chemotherapy should be alerted to this report and closely monitor whether ILD develops in patients treated with these agents.
Figures and Tables
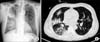 | Figure 1Chest X-ray (A) and chest high resolution computed tomography (B) on admission showing multifocal patchy consolidation and ground glass opacities on both lungs, mainly on the right lung. |
References
1. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009; 22:191–197.
2. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000; 355:1041–1047.
3. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947.
4. Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004; 15:933–939.
5. Fukuoka M, Niitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, et al. A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer. J Clin Oncol. 1992; 10:16–20.
6. Pasetto LM, Jirillo A, Iadicicco G, Rossi E, Paris MK, Monfardini S. FOLFOX versus FOLFIRI: a comparison of regimens in the treatment of colorectal cancer metastases. Anticancer Res. 2005; 25:563–576.
7. Shimura T, Fuse N, Yoshino T, Minashi K, Tahara M, Doi T, et al. Clinical features of interstitial lung disease induced by standard chemotherapy (FOLFOX or FOLFIRI) for colorectal cancer. Ann Oncol. 2010; 21:2005–2010.
8. Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, et al. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol. 1997; 15:2910–2919.
9. Yoshii N, Suzuki T, Nagashima M, Kon A, Kakihata K, Gemma A. Clarification of clinical features of interstitial lung disease induced by irinotecan based on postmarketing surveillance data and spontaneous reports. Anticancer Drugs. 2011; 22:563–568.
10. Takano T, Ohe Y, Kusumoto M, Tateishi U, Yamamoto S, Nokihara H, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004; 45:93–104.
11. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006; 24:2549–2556.
12. Michielin O, Udry E, Periard D, Matzinger O, Lobrinus JA, Stupp R. Irinotecan-induced interstitial pneumonia. Lancet Oncol. 2004; 5:322–324.
13. Madarnas Y, Webster P, Shorter AM, Bjarnason GA. Irinotecan-associated pulmonary toxicity. Anticancer Drugs. 2000; 11:709–713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download