Abstract
Anaplastic lymphoma kinase (ALK) rearrangement, is a kind of driver mutation, accounts for 3%-5% of non-small cell lung cancer (NSCLC). NSCLC patients harboring ALK fusion genes have distinct clinical features and good response to ALK inhibitors. Metastasis from lung cancer to the ovary has rarely been known. We report a case of a 54-year-old woman with bilateral ovarian metastases from ALK rearranged NSCLC. She underwent bilateral salpingo-oophorectomy for ovary masses, which were progressed after cytotoxic chemotherapy although primary lung mass was decreased. Histopathological examination of the ovary tumor showed characteristic adenocarcinoma patterns of the lung and ALK rearrangement.
Anaplastic lymphoma kinase (ALK) gene rearrangement has emerged as an important driver mutation in non-small cell lung cancer (NSCLC) since echinoderm microtubule associated protein-like 4 (EML4)-ALK fusion gene was discovered in 20071. ALK gene rearrangement is found in approximately 3%-7% of NSCLC. ALK fusion genes are more frequently found in patients with adenocarcinoma histology, younger age, and light or never smoking history and they are sensitive to the ALK inhibitors2,3. Although EML4-ALK translocations tend to occur in patients with more advanced NSCLC2, ovarian metastasis originating from lung cancer is extremely rare. Here, we report a case of a 54-year-old woman with bilateral ovarian metastases from ALK rearranged NSCLC.
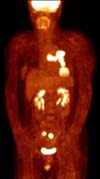
A 54-year-old woman with a 2.5 pack-year smoking history presented with cough and dyspnea. Computed tomography (CT) scan showed a 5.4- and 3.3-cm sized left lower lobe masses with left hilar and subcarinal lymphadenopathies (Figure 1). Positron emission tomography-computed tomography (PET-CT) showed increased fluorodeoxyglucose uptake in two left lower lobe masses and bilateral ovaries (Figure 2). Brain magnetic resonance imaging (MRI) revealed disseminated brain metastasis. CT-guided percutaneous transthoracic needle biopsy of the lung mass confirmed adenocarcinoma and the tumor showed marked ALK protein expression by immunohistochemistry (IHC). Fluorescent in situ hybridization (FISH) analysis for ALK translocation revealed also positive. However, an analysis of biopsy specimen showed no evidence of a preexisting mutation in epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma viral oncogene (KRAS).
She received first-line cytotoxic chemotherapy consisting of pemetrexed (500 mg/m2) and cisplatin (60 mg/m2) instead of crizotinib with whole brain radiation therapy to a total dose of 3,000 cGy in 10 fractions on account of financial reasons. Approximately 10 days after starting first-line chemotherapy, the patient presented fever and cough. Initially the patient was treated with antibiotics for presumed pneumonia. Her symptoms progressed through 3 consecutive days and were accompanied by left pleuritic chest pain. High resolution CT was performed and demonstrated progression of pulmonary masses. Size of primary tumor and additional tumor was increased from 5.4 cm and 3.3 cm to 6.0 cm and 3.6 cm, respectively. She was treated with erlotinib 150 mg/day as second-line therapy and palliative radiation therapy to lung masses in left lower lobe. A total dose of 3,500 cGy was delivered in 14 fractions.
After 3 weeks of erlotinib as second-line therapy, abdominal CT scan showed increased sized bilateral ovarian masses (right, 2 to 4.1 cm; left, 2.7 to 5 cm) while pulmonary masses (primary mass, 6.0 to 4.9 cm; additional mass, 3.6 to 2.9 cm) and metastatic mediastinal lymph nodes were decreased in size on chest CT scan. She underwent laparoscopic bilateral salpingo-oophorectomy to differentiate synchronous bilateral ovarian cancers and tumors metastatic to the both ovaries. Microscopic examination of both ovaries revealed metastatic adenocarcinoma from the lung (Figure 3). IHC and FISH analyses for ALK translocation were both positive (Figure 4).
Five weeks after the initiation of erlotinib, rapid progression to adrenal and pancreatic metastases was detected on the follow-up PET-CT although primary lung mass and disseminated brain metastases that had been radiation treatment fields revealed partial improvement. She stopped taking erlotinib and changed to take third-line chemotherapy consisting of gemcitabine (1,000 mg/m2) and carboplatin (4 area under the curve).
After four cycles of third-line chemotherapy, chest CT showed increased size of pulmonary masses, metastatic mediastinal lymph nodes and left adrenal mass. In addition, brain MRI showed progression of the multiple brain metastases. Eventually, the patient discontinued chemotherapy owing to unacceptable toxic effects and no further clinical benefit.
Fusion of the ALK with the EML4 is found in approximately of 3%-7% of all NSCLC. ALK rearranged NSCLC has unique clinical and molecular features. Patients with ALK rearranged NSCLC are relatively young, never or former light smoker and have histology of adenocarcinoma2. Crizotinib, ALK inhibitor is preferred as the initial therapy of advanced ALK-positive lung cancer4. In addition, second-generation ALK inhibitors or heat shock protein 90 inhibitors are under clinical studies for ALK-positive lung cancer patients, because of acquired resistance to crizotinib like as NSCLC patients harboring EGFR mutation.
However, in the present case, the patient could not afford to receive treatment with crizotinib for financial problems at the start and underwent pemetrexed based chemo-radiation therapy. The choice of specific chemotherapy agent or regimen for ALK-positive lung cancer is accordance with histologic type in a similar way of other forms of NSCLC. One large multicenter retrospective study showed a similar level of progression free survival on pemetrexed or nonplatinum/pemetrexed combinations in ALK-positive and ALK-negative lung cancer patients4. In addition, subgroup analysis of crizotinib versus either pemetrexed or docetaxel in the phase III study (PROFILE 1007) of advanced ALK-positive NSCLC showed pemetrexed's superior efficacy over docetaxel. Median progression free survival was longer on pemetrexed (4.2 months) than docetaxel (2.6 months) and 1-year progression free survival rates were 16% on pemetrexed and 6% on docetaxel5.
This case showed that primary lung mass and metastatic brain tumors were relatively sensitive to radiation therapy, despite rapid development of distant metastases. Hayashi et al.6 also reported a NSCLC with ALK rearrangement case who showed complete response to radiation therapy of cystic brain metastasis.
Metastatic ovarian tumors are not uncommon, which account for approximately 10%-30% of all ovarian cancers7,8. The common primary sites are colon, stomach, appendix, breast and pancreas7. However, ovarian metastasis from lung cancer is extremely rare, it accounts for only 0.3%-0.4% of metastatic ovarian tumors9,10. Although some retrospective analyses reported that patients with metastatic NSCLC harboring ALK rearrangement might be correlated with increased risk of pericardium and pleural metastases11,12. Other than common sites of metastasis of lung cancer such as bones, liver adrenal glands and brain, metastases to bilateral ovaries of ALK rearranged NSCLC may not be a coincidence. Further research is necessary to define a distinct metastatic behavior of ALK rearranged NSCLC such as bilateral ovaries and the effectiveness of radiation therapy to the ALK rearranged NSCLC. Differential diagnosis between primary and metastatic ovarian tumors is needed due to different treatment modality such as cytoreductive surgery or palliative chemotherapy with appropriate regimen. After second-line chemotherapy, this case report showed mixed response to chemotherapy; the size of primary lung lesion decreased however that of both ovarian lesions increased. Therefore to rule out synchronous double primary ovarian cancer, we decided to perform laparoscopic bilateral salpingo-oophorectomy aggressively. There is no available noninvasive diagnostic tool for the differential diagnosis between primary and metastatic ovarian tumors, and surgery is inevitably needed to make an accurate diagnosis and plan appropriate treatment strategy.
Figures and Tables
Figure 1
Computed tomography scan showing a 5.4-cm- (A) and 3.3-cm-sized (B) left lower lobe masses with left hilar (C) and subcarinal lymphadenopathies.

Figure 2
Bilateral ovarian metastases of lung cancer at the initial positron emission tomography-computed tomography scan showing hypermetabolic activity in both ovaries (maximum standardized uptake value: right 15.7, left 13.4).
References
1. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–566.
2. Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010; 46:1773–1780.
3. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703.
4. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368:2385–2394.
5. Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs. 2013; 73:2031–2051.
6. Hayashi H, Okamoto I, Tanizaki J, Tanaka K, Okuda T, Kato A, et al. Cystic brain metastasis in non-small-cell lung cancer With ALK rearrangement. J Clin Oncol. 2014; 32:e122–e124.
7. Alvarado-Cabrero I, Rodriguez-Gomez A, Castelan-Pedraza J, Valencia-Cedillo R. Metastatic ovarian tumors: a clinicopathologic study of 150 cases. Anal Quant Cytopathol Histpathol. 2013; 35:241–248.
8. Jung YE, Lee JW, Kim BG, Bae DS. Ovarian metastasis from pulmonary adenocarcinoma. Obstet Gynecol Sci. 2013; 56:341–344.
9. Fujiwara K, Ohishi Y, Koike H, Sawada S, Moriya T, Kohno I. Clinical implications of metastases to the ovary. Gynecol Oncol. 1995; 59:124–128.
10. Irving JA, Young RH. Lung carcinoma metastatic to the ovary: a clinicopathologic study of 32 cases emphasizing their morphologic spectrum and problems in differential diagnosis. Am J Surg Pathol. 2005; 29:997–1006.
11. Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012; 118:4502–4511.
12. Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, Wampfler J, et al. Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol. 2012; 7:90–97.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download