Abstract
Segniliparus species is a novel genus that is reported to be the new emerging respiratory pathogens. Here, we report a very rare case of S. rugosus pulmonary infection in an immunocompetent patient with non-cystic fibrosis. The organism was identified by 16S rRNA gene sequencing. The patient was successfully treated with antibiotics.
Segniliparus is a novel genus consisting of two species, S. rugosus and S. rotundus, that was first described in 2005 as a distinct genus isolated from human sources as a group of rapidly growing acid-fast bacilli (AFB)1. Since then, cases of Segniliparus infection have been reported in bronchiectasis patients with2,3 or without4 cystic fibrosis. In addition, Segniliparus isolation was recently even reported in a non-bronchiectasis patient in South Korea5. Here, we report a very unusual case of S. rugosus pulmonary infection in an immunocompetent, non-cystic fibrosis patient.
A 47-year-old woman was referred to our hospital for a recently aggravating cough lasting for 3 months. About 20 years earlier, she had been treated for pulmonary tuberculosis. The patient had experienced chronic coughing for the previous 3 years. A chest X-ray and chest computed tomography (CT) taken 2 years previously revealed centrilobular nodules and bronchiectatic changes (Figure 1). Although AFB cultures yielded Mycobacterium abscessus growth on several occasions at that time, she did not undergo treatment for M. abscessus because her symptoms were mild and CT did not reveal severe changes. She therefore underwent regular follow-up visits. Although she had been relatively well and her chest X-ray did not show significant changes during the approximately 2-year follow-up, she experienced an aggravation of her symptoms.
On physical examination, the patient was alert and in no distress. Her body temperature was 36.3℃, blood pressure was 106/70 mm Hg, pulse was 72 beats per minute with a regular rhythm, and respiratory rate was 20 breaths per minute. Bronchial breathing sounds and inspiratory rhonchi were heard in the bilateral anterior chest. A complete blood count revealed a white blood cell count of 6,500/mm3 (64% neutrophils), hemoglobin of 13.7 g/dL, and platelets of 239,000/mm3. Her C-reactive protein concentration was 1.7 mg/dL. Routine chemical laboratory data were all within normal ranges. The patient was negative for antibodies to human immunodeficiency virus.
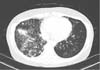
Compared with a scan taken 2 years previously, her chest CT showed an increased amount and extent of multifocal small nodules as well as newly developed consolidation (Figure 2). Multiple specimens were examined for mycobacteria. Although AFB stains were positive in sputum examination using both auramine-rhodamine fluorescent and Ziehl-Neelsen methods, no organism was identified using an ACE detection kit (Seegene, Seoul, Korea) that can detect both Mycobacterium tuberculosis (MTB) and non-tuberculosis mycobacterium (NTM).
To identify a source of infection other than MTB or NTM, 16S rRNA polymerase chain reaction (PCR) was performed using primers specific for the 9-806 bp (8FPL: 5'-AGT TTG ATC CTG GCT CAG-3', 806R: 5'-GGA CTA CCA GGG TAT CTA AT-3') and 515-1,390 bp (515FPL: 5'-TGC CAG CAG CCG CGG TAA-3', 13B: 5'-AGG CCC GGG AAC GTA TTC AC-3') segments according to previously published methods6. Purified PCR products were directly sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). According to a search using the Basic Local Alignment Search Tool (BLAST) database (http://www.ncbi.nlm.nih.gov/blast/) and EzTaxon-e (http://eztaxon-e.ezbiocloud.net/), the sequence of this isolate exhibited 100% homology (1,316 of 1,316 bp) with that of S. rugosus ATCC BAA-974T and 98.8% homology (1,300 of 1,316 bp) with that of S. rotundus DSM 44985T. In line with the Clinical Laboratory Standard Institute guidelines, the organism was identified as S. rugosus7.
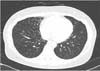
Drug susceptibility testing for S. rugosus failed several times due to contamination and inadequate growth. Antibiotic treatment for S. rugosus was initiated with oral clarithromycin at 1,000 mg/day and moxifloxacin at 400 mg/day for 6 months, intravenous amikacin 15 mg/kg/day for 3 months, and intravenous imipenem/cilastatin 2,250 mg/day for 1 month. The patient's symptoms improved rapidly after initiation of treatment and culture conversion was seen after 1 month. Treatment was completed after 6 months at which time a CT scan was taken and showed an improvement in lung lesions (Figure 3). The patient has since been on a regular follow-up for 1 year at our hospital without.
We have here described here a very rare case of S. rugosus infection in an immunocompetent patient with non-cystic fibrosis. Only a few studies to date have reported S. rugosus infection in patients with cystic fibrosis2,3 or simple S. rugosus isolation from a patient with radiographic features mimicking those of NTM5. To our knowledge, our current report is the first to describe a case of infection due to S. rugosus in a non-cystic fibrosis patient that was successfully treated with antibiotics.
In our current patient, S. rugosus infection seemed to have occurred in damaged and inflamed bronchus caused by previous infection with M. abscessus. The patient was a 47-year-old woman with underlying bronchiectasis, which coincides with the age and gender predilection for the nodular bronchiectatic type of NTM lung disease. Given that Segniliparus can be easily confused with nonchromogenic rapid-growing mycobacteria (RGM) under the microscope due to their similar acid-fast staining properties, our case could have simply been mislabeled as aggravated M. abscessus infection. Thus, along with the other case reports2,3,4,5, our case indicates that physicians should be aware of the possibility of infection with this rare but emerging organism if Mycobacterium species is not detected with AFB culture or PCR methods from positive AFB smear specimens. Molecular diagnostic methods using sequencing analysis of 16S rRNA genes enable the accurate identification of S. rugosus.
Regarding the treatment of Segniliparus species, only limited information is available. For the first case report of the S. rugosus in cystic fibrosis patient, successful treatment was done using imipenem (1 g IV, every 6 to 8 hours for 6 months), oral rifampin (150 mg once daily), and oral sulfamethoxazole-trimethoprim (80 mg) according to the susceptibility test results2. And, in case of S. rotundus in Korea, favorable outcome were noted using oral clarithromycin (1,000 mg/day) and ciproproxacin (1,000 mg/day) for 2 months of treatment4. Even effective treatment for RGM has not been established, 2007 American Thoracic Society (ATS)/Infectious Disease Society of America (IDSA) guideline suggested that clarithromycin combined with amikacin, cefoxitin or imipenem for 2 to 4 months usually produced clinical and microbiologic improvement8. Since we were unable to perform drug susceptibility testing, we treated our patient empirically in accordance with ATS/IDSA guideline for RGM and considering previous two case study regimen with a successful response2,4,8. Further studies are required to establish the optimal regimen for this organism and its duration.
Figures and Tables
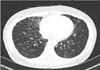
Figure 1
An initial chest computed tomography scan of the studied patient showing bronchiectatic changes with small centrilobular nodules in the lingular division of the left upper lobe, the right middle lobe, the right lower lobe, and the left lower lobe.
References
1. Butler WR, Floyd MM, Brown JM, Toney SR, Daneshvar MI, Cooksey RC, et al. Novel mycolic acid-containing bacteria in the family Segniliparaceae fam. nov., including the genus Segniliparus gen. nov., with descriptions of Segniliparus rotundus sp. nov. and Segniliparus rugosus sp. nov. Int J Syst Evol Microbiol. 2005; 55(Pt 4):1615–1624.
2. Butler WR, Sheils CA, Brown-Elliott BA, Charles N, Colin AA, Gant MJ, et al. First isolations of Segniliparus rugosus from patients with cystic fibrosis. J Clin Microbiol. 2007; 45:3449–3452.
3. Hansen T, Van-Kerckhof J, Jelfs P, Wainwright C, Ryan P, Coulter C. Segniliparus rugosus infection, Australia. Emerg Infect Dis. 2009; 15:611–613.
4. Koh WJ, Choi GE, Lee SH, Park YK, Lee NY, Shin SJ. First case of Segniliparus rotundus pneumonia in a patient with bronchiectasis. J Clin Microbiol. 2011; 49:3403–3405.
5. Choi SM, Kang HJ, Jeong YJ, Lim JH, Choe WS, Hwang SH, et al. First isolation of Segniliparus rugosus from a patient with radiologic features similar to non-tuberculous mycobacteriosis. Tuberc Respir Dis. 2012; 72:82–87.
6. Relman DA. Universal bacterial 16sRNA amplification and sequencing. In : Persing DH, Smith TF, Tenover FC, White TJ, editors. Diagnostic molecular microbiology: principles and applications. Rochester: Mayo Foundation;2013. p. 489–495.
7. Petti CA, Bosshard PP, Brandt ME, Clarridge JE, Feldblyum TV, Foxall P, et al. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing. approved guideline. MM 18-A. Wayne: Clinical and Laboratory Standards Institute;2008.
8. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download