Abstract
We present a case of an unusual infectious complication of a ruptured mediastinal abscess after endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), which led to malignant pleural effusion in a patient with stage IIIA non-small-cell lung cancer. EBUS-TBNA was performed in a 48-year-old previously healthy male, and a mediastinal abscess developed at 4 days post-procedure. Video-assisted thoracoscopic surgery was performed for debridement and drainage, and the intraoperative findings revealed a large volume pleural effusion that was not detected on the initial radiographic evaluation. Malignant cells were unexpectedly detected in the aspirated pleural fluid, which was possibly due to increased pleural permeability and transport of malignant cells originating in a ruptured subcarinal lymph node from the mediastinum to the pleural space. Hence, the patient was confirmed to have squamous cell lung carcinoma with malignant pleural effusion and his TNM staging was changed from stage IIIA to IV.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a useful diagnostic method for evaluation of lung cancer1. EBUS-TBNA is generally considered a safe procedure to access mediastinal structures compared with mediastinoscopy and video-assisted thoracic surgery2. Based on safety and tolerance, EBUS-TBNA is typically performed under conscious sedation and local anesthesia3.
Although complications from EBUS-TBNA are rare, serious complications such as mediastinitis, hemorrhage, and tumor rupture have been reported occasionally with the recent widespread use of EBUS-TBNA4. However, no study has reported a ruptured mediastinal abscess that developed after EBUS-TBNA and resulted in a potential pleural seeding metastasis. Herein, we report an unusual infectious complication of a ruptured mediastinal abscess after EBUS-TBNA that led to malignant pleural effusion in a patient with stage IIIA non-small-cell lung cancer.
A 48-year-old male visited our clinic with a 1-month history of cough. He had been healthy and had no specific family history. An initial chest radiograph showed a mass-like lesion in the right lower lung zone, and a subsequent chest computed tomography (CT) scan confirmed a 5.1-cm-sized right lung mass with enlarged lymph nodes at the right hilar, subcarinal, and right lower paratracheal area, which was highly suspected to be primary lung cancer (Figure 1A, B). Positron emission tomography and magnetic resonance imaging of the brain detected no distant metastasis; therefore, EBUS-TBNA (BF-UC260F-OL8; Olympus, Tokyo, Japan) was performed for both pathologic diagnosis and nodal staging of the suspected lung cancer according to the American College of Chest Physicians evidence-based clinical practice guidelines (Figure 1C, D)5. Ultrasound findings showed that the subcarinal and right lower paratracheal lymph nodes were enlarged (19×21 mm and 10×16 mm, respectively) with discrete margins and central hypoechogenicity. During needle aspiration, lymph node consistency was supposedly soft and tender. Eventually, core tissues with necrotic materials were obtained from the subcarinal and right lower paratracheal lymph nodes. No immediate EBUS-TBNA-related complication was detected, and the patient tolerated the procedure well under conscious sedation. Core tissues obtained from EBUS-TBNA were revealed to be squamous cell carcinoma with extensive necrosis (Figure 1E, F). Finally, the patient was diagnosed with stage IIIA (T2bN2M0) squamous cell carcinoma of the lung. He was planned to receive neoadjuvant concurrent chemoradiotherapy but was subsequently considered for curative surgery.
For prompt initiation of concurrent chemoradiotherapy, the patient remained hospitalized until the pathologic result of EBUS-TBNA was confirmed. He complained of a burning chest pain with fever of up to 38.9℃ 4 days after EBUS-TBNA. Laboratory examinations revealed that the blood leukocyte count was 15,330/µL with a differential of 79.9% neutrophils (normal range, 40-73%) and C-reactive protein level had increased to 5.91 mg/dL (normal range, 0-0.5 mg/dL). A chest radiograph showed normal findings; therefore, a subsequent chest CT scan was performed to identify a suspicious complication possibly related to EBUS-TBNA. The chest CT scan showed a significantly increased ill-defined soft tissue density and fluid collection in the mediastinum with an increase in the size of the subcarinal lymph node (Figure 2). The patient was started on empirical antibiotic treatment including piperacillin/tazobactam and vancomycin for management of the mediastinal infection caused by EBUS-TBNA.
He complained of severe chest pain and dyspnea with tachycardia (110-130 beats per minute), hypoxia (peripheral oxygen saturation<90%) and increased C-reactive protein level up to 31.31 mg/dL 2 days later. The patient was referred for emergency surgical debridement and drainage using video-assisted thoracoscopic surgery. The intraoperative findings during video-assisted thoracoscopic surgery revealed a large volume of pleural effusion, which was not found on the initial chest CT scan and positron emission tomography, and aspirated pleural fluid was sent for a microbiological and cytological examination. After a thoracoscope was introduced through the mediastinal space, a ruptured subcarinal lymph node with a leaking abscess was found. The mediastinal pleura and subcarinal and right lower paratracheal lymph nodes were debrided, and the patient recovered fully after 2 weeks of additional antibiotic treatment.
No significant microorganisms were isolated in culture specimens of the debrided material. The resected subcarinal lymph node revealed metastatic squamous cell carcinoma associated with the abscess (Figure 3A, B); however, only inflammatory changes on the mediastinal pleural were noted, without evidence of metastatic involvement (Figure 3C). A cytological analysis of the drained pleural fluid showed metastatic squamous cell carcinoma (Figure 3D); however, metastatic lesions were not detected on the previous chest CT scan or positron emission tomography before development of the mediastinal abscess. After recovery from mediastinitis, chest CT was performed again, and no evidence of pleural effusion or pleural seeding metastasis was shown. However, malignant cells were verified at the drained pleural fluid during video-assisted thoracoscopic surgery; therefore, we could not exclude potential pleural seeding metastasis. Accordingly, the patient was diagnosed with squamous cell lung cancer with malignant pleural effusion and his cancer staging was advanced from stage IIIA (T2bN2M0) to IV (T2bN2M1a). Four weeks after video-assisted thoracoscopic surgery, systemic chemotherapy was initiated in accordance with the protocol for management of stage IV non-small-cell lung cancer.
EBUS-TBNA is an accurate and minimally invasive technique for evaluating mediastinal lesions; however, serious complications have been reported occasionally due to its increased use4,6,7. Reported infectious complications include mediastinitis, pneumonia, pericarditis, cyst infection and sepsis, with a 0.19% incidence.
Interestingly, our patient was initially diagnosed with stage IIIA lung cancer without pleura metastasis and pleural effusion; however, malignant pleural effusion was documented after development of the EBUS-TBNA-related mediastinal abscess. Moreover, the mediastinal pleura obtained through video-assisted thoracoscopic surgery showed only inflammatory changes. We estimated that increased pleural membrane permeability due to pleural inflammation resulted in the transportation of malignant cells that originated from a ruptured subcarinal lymph node to the pleural space. Mohammed et al.8 reported that bacterial infection of lung parenchyma causes a permeability change in the pleural mesothelium. Inflammation of the pleural membrane induces vascular endothelial growth factor release, which downregulates catenins and cadherin8,9. Subsequently, pleural permeability and movement of cells across the intermesothelial gap increases. Accordingly, increased permeability of the mediastinal pleura, which resulted from the mediastinal abscess, could have led to the development of malignant pleural effusion in the present case.
Thus far, no consensus on the use of prophylactic antibiotics for EBUS-TBNA has been established. Infectious complications have been reported following EBUS-TBNA on cystic or necrotic lesions4,10. In addition, prophylactic antibiotics are recommended for endoscopic ultrasound-guided fine-needle aspiration on cystic lesions11. From the previous reports of infectious complications after EBUS-TBNA and guidelines for endoscopic ultrasound-guided fine needle aspiration, we suggest that, in necrotic or cystic lesions, EBUS-TBNA should be performed carefully and prophylactic antibiotics should be considered.
In conclusion, this case is the first report of malignant pleural effusion resulting from EBUS-TBNA-related infectious complications. Unfortunately, TNM staging of our patient was changed from stage IIIA (T2bN2M0) to IV (T2bN2M1a). The patient was initially scheduled for curative surgery after neoadjuvant concurrent chemoradiotherapy; however, systemic chemotherapy was initiated in accordance with the protocol for management of stage IV lung cancer.
Figures and Tables
Figure 1
A 48-year-old male with squamous cell lung cancer and metastatic involvement of subcarinal (white arrows) and right lower paratracheal (black arrows) lymph nodes. (A, B) Initial chest computed tomography scan shows subcarinal and right lower paratracheal lymph node enlargement. (C, D) An endobronchial ultrasound image of the subcarinal and right lower paratracheal lymph nodes shows hypoechoic texture, a round shape, and well-demarcated borders. (E, F) The tumor cells formed sheets of hyperchromatic cells with a high nuclear-cytoplasmic ratio and focal keratinization. The specimen has a necrotic and hemorrhagic background (H&E stain, ×400).

Figure 2
Computed tomography scan obtained 4 days after endobronchial ultrasound-guided transbronchial needle aspiration demonstrates an increase in size of the subcarinal lymph node with ill-defined soft tissue density and fluid collection in the mediastinum, suggesting mediastinitis associated with subcarinal abscess leakage.
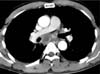
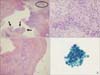
Figure 3
Microscopic findings of the specimens obtained via video-assisted thoracoscopic surgery. (A) The subcarinal lymph node is mostly replaced with an abscess and clusters of squamous cell carcinoma are present (black arrows) in the abscess (H&E stain, ×40). (B) Squamous cell carcinoma is present in the background of the abscess in a high power field of the circled area in A (H&E stain, ×400). (C) Mediastinal pleura show chronic inflammation with fibrosis (H&E stain, ×100). (D) Cytological smear of the pleural fluid shows squamous cell carcinoma. The tumor cells have a high nuclear-cytoplasmic ratio, irregular nuclear membrane, dense cytoplasm, and well-defined borders (Papanicolaou stain, ×1,000).
Acknowledgements
This study was supported by a clinical research grant from the Biomedical Research Institute, Pusan National University Hospital (2014).
References
1. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009; 6:180–186.
2. Evison M, Crosbie PA, Martin J, Bishop P, Doran H, Joseph L, et al. EBUS-TBNA in elderly patients with lung cancer: safety and performance outcomes. J Thorac Oncol. 2014; 9:370–376.
3. Kokkonouzis I, Strimpakos AS, Lampaditis I, Tsimpoukis S, Syrigos KN. The role of endobronchial ultrasound in lung cancer diagnosis and staging: a comprehensive review. Clin Lung Cancer. 2012; 13:408–415.
4. Asano F, Aoe M, Ohsaki Y, Okada Y, Sasada S, Sato S, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res. 2013; 14:50.
5. Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143:5 Suppl. 7S–37S.
6. Lee JW, Kim WJ, Park CW, Kang HW, Ban HJ, Oh IJ, et al. Endotracheal tuberculous granuloma formation following endobronchial ultrasound transbronchial needle aspiration. Intern Med. 2013; 52:1207–1210.
7. Kinsey CM, Arenberg DA. Endobronchial ultrasound-guided transbronchial needle aspiration for non-small cell lung cancer staging. Am J Respir Crit Care Med. 2014; 189:640–649.
8. Mohammed KA, Nasreen N, Hardwick J, Logie CS, Patterson CE, Antony VB. Bacterial induction of pleural mesothelial monolayer barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001; 281:L119–L125.
9. Mohammed KA, Nasreen N, Hardwick J, Van Horn RD, Sanders KL, Antony VB. Mycobacteria induces pleural mesothelial permeability by down-regulating beta-catenin expression. Lung. 2003; 181:57–66.
10. Kang HJ, Hwangbo B. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration. Tuberc Respir Dis. 2013; 75:135–139.
11. Polkowski M, Larghi A, Weynand B, Boustiere C, Giovannini M, Pujol B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012; 44:190–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download