Abstract
Valproic acid is one of the most common antiepileptic drugs used for the treatment of several seizure disorders. A 20-year-old man presented with a sudden decline of consciousness. He had a neurosurgery operation for intracranial and intraventricular hemorrhage. Following surgery, antiepileptic medication was administered to the patient in order to control his seizure events. On valproic acid treatment, he began to complain of fever and dyspnea. His symptoms persisted despite receiving empirical antibiotic treatment. All diagnostic tests for infectious causes were negative. A high-resolution computed tomography scan of the chest revealed predominantly dependent consolidation and ground-glass opacities in both lower lobes. The primary differential was drug associated with interstitial lung disease. Therefore, we discontinued valproic acid treatment and began methylprednisolone treatment. His symptoms and radiologic findings had significantly improved after receiving steroid therapy. We propose that clinicians should be made aware of the potential for valproic acid to induce lung injury.
Valproic acid is one of the most common antiepileptic drugs used for the treatment of several seizure disorders. It is approved by the Food and Drug Administration for use in the treatment of manic episodes associated with bipolar disorder, as an adjunctive therapy in multiple types of seizures, and for the prophylaxis of migraine headaches.
Valproic acid is known to induce several adverse effects involving the hepatobiliary, renal, neurologic, hematologic, cardiovascular, gastrointestinal, and metabolic systems1. However, adverse effects of valproic acid on the pulmonary system, including acute eosinophilic effusion or diffuse alveolar hemorrhage, have rarely been reported. Further, interstitial lung disease caused by valproic acid has not been previously reported. Herein, we describe a patient with drug-induced interstitial pneumonitis caused by valproic acid administered to treat seizures.
A 20-year-old man presented with a sudden decline of consciousness. He had no significant past medical history, and had never smoked.
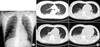
On admission, the patient's vital signs were as follows: blood pressure, 130/80 mm Hg; pulse rate, 88 beats per minute; respiratory rate, 16 breaths per minute; and body temperature, 36.7℃. A clear breathing sound without fine crackles and no wheezing was heard following chest auscultation of both lower lung fields. The patient's laboratory test results were as follows: hemoglobin, 11.8 g/dL; white blood cell count, 8,330 cells/µL (neutrophils 77.2%, lymphocytes 8.8%, monocytes 9.1%, eosinophils 4.8%, and basophils 0.1%); and platelet count, 172,000 cells/µL. The patient's serum biochemistry test results revealed aspartate aminotransferase 23 IU/L, alanine aminotransferase 15 IU/L, total bilirubin 0.4 mg/dL, alkaline phosphatase 51 IU/L, total protein 6.7 g/dL, albumin 3.9 g/dL, blood urea nitrogen 13.8 mg/dL, creatinine 0.7 mg/dL, total cholesterol 125 mg/dL, procalcitonin 0.61 ng/mL, and C-reactive protein 0.83 mg/dL. Arterial blood gas analysis of the fraction of inspired oxygen (FiO2) was 0.28, and revealed the following: pH, 7.481; partial pressure of oxygen (PaO2), 165.0 mm Hg; partial pressure of carbon dioxide (PaCO2), 26.1 mm Hg; bicarbonate (HCO3-), 19.2 mEq/L; and saturation level of oxygen (SaO2), 99.5%. Chest radiography showed no active parenchymal lesion (Figure 1). A brain computed tomography revealed intracranial and intraventricular hemorrhage. The patient was admitted to the department of neurosurgery, and a surgical procedure was performed.
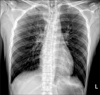
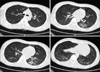
Following surgery, the patient was administered anti-hypertensive medication (nicardipine, 2 mg/hr, intravenous) and antibiotics (ceftriaxone, 2 g every 24 hours), as well as intravenous vitamin K (10 mg, every 8 hours) and tranexamic acid (500 mg, every 8 hours) to control bleeding. On day six following hospitalization, antiepileptic medication was administered to the patient to control his seizure events (sodium valproate, 400 mg, every 8 hours). On day fourteen following hospitalization, the patient's neurologic symptoms were improved. However, he began complaining of fever and dyspnea. The patient's vital signs were as follows: blood pressure, 140/80 mm Hg; pulse rate, 118 beats per minute; respiratory rate, 28 breaths per minute; and body temperature, 39.1℃. A coarse breathing sound with fine crackles and no wheezing was heard following chest auscultation of both lower lung fields. The patient's laboratory test results were as follows: hemoglobin, 12.2 g/dL; white blood cell count, 19,480 cells/µL (neutrophils 91.6%, lymphocytes 3.0%, monocytes 4.6%, eosinophils 0.6%, and basophils 0.2%); and platelet count, 282,000 cells/µL. The patient's serum biochemistry test results revealed aspartate aminotransferase 38 IU/L, alanine aminotransferase 25 IU/L, total bilirubin 0.5 mg/dL, alkaline phosphatase 43 IU/L, total protein 6.6 g/dL, albumin 3.8 g/dL, BUN 20.1 mg/dL, creatinine 0.7 mg/dL, total cholesterol 142 mg/dL, procalcitonin 0.61 ng/mL, and C-reactive protein 12.62 mg/dL. Arterial blood gas analysis of the fraction of inspired oxygen (FiO2) was 0.28, and revealed the following: pH, 7.506; partial pressure of oxygen (PaO2), 55.0 mm Hg; partial pressure of carbon dioxide (PaCO2), 28.6 mm Hg; bicarbonate (HCO3-), 22.4 mEq/L; and saturation level of oxygen (SaO2), 89.0%. Chest radiography revealed consolidation in both lower lung fields (Figure 2A). In addition, a high-resolution computed tomography (HRCT) scan of the chest revealed predominantly dependent consolidation with air bronchogram and ground-glass opacities in both lower lobes (Figure 2B).
The patient required mechanical ventilation support due to the rapid and progressive aggravation of clinical symptoms. Hence, administration of empirical antibiotic therapy for hospital-acquired pneumonia was commenced (meropenem, 1 g every 8 hours, and levofloxacin, 750 mg every 24 hours). Despite two days of antibiotic therapy, the patient's radiologic and clinical findings persisted. Consequently, bronchoscopy with bronchoalveolar lavage was performed at the superior segment of the right lower lobe. Bronchoalveolar lavage fluid differential cell counts were as follows: white blood cell, 1,780 cells/µL (neutrophils 83%, lymphocytes 15%, macrophage 1%, eosinophils 1%, and basophils 0%); and red blood cell, 11,350 cells/µL. The bronchoscopic washing specimens were negative for Mycobacterium tuberculosis, as determined by the acid-fast bacilli smear, polymerase chain reaction, and microbial cultures. Based on the clinical symptoms and the low procalcitonin level, a diagnosis of drug-induced interstitial lung disease (DILD) was suspected. Thus, valproic acid treatment was discontinued, and treatment with methylprednisolone 50 mg (1 mg/kg of body weight) was initiated. After three days of steroid therapy, the patient's symptoms and radiological findings had significantly improved. The dosage of methylprednisolone was gradually decreased to 10 mg daily for two weeks. Two weeks later, a HRCT of chest revealed complete remission, and indicated no sign of bilateral infiltrates (Figure 3).
Valproic acid is one of the most common antiepileptic drugs used for the treatment of several seizure disorders. However, valproic acid is known to induce several adverse effects involving the hepatobiliary, renal, neurologic, hematologic, cardiovascular, gastrointestinal, and metabolic systems. Adverse pulmonary effects associated with valproic acid, such as diffuse alveolar hemorrhage or eosinophilic effusion, have rarely been reported. Additionally, we found no reports in the literature of any association between valproic acid administration and interstitial lung disease2,3,4.
Several types of drugs can cause DILD. However, the associated incidence of DILD varies for each individual drug. DILD may be mild to progressive, and in its more severe manifestation, DILD may result in respiratory failure and acute respiratory distress syndrome. Further, DILD may develop within the first few days of treatment, or may not manifest until several years after treatment.
Diagnosis of DILD generally depends on a definite temporal association between exposure to the causative agent, and the development of respiratory signs and symptoms. Specific markers, histological findings, and diagnostic clinical features are generally unremarkable in DILD5,6. Difficulties arise when signs and symptoms develop after the drug is discontinued, rather than during treatment, or when no improvement follows discontinuation of the drug. Making a timely and accurate diagnosis of DILD is very important to ensure a favorable outcome7. Most importantly, for an accurate diagnosis, other causes of lung damage such as presence of infectious disease must be excluded8,9.
A pathological examination was not performed on the patient in the current case report. However, infectious pathogens were excluded as the cause of interstitial pneumonia, as a microbial culture was negative for infectious bacteria. In addition, the patient's symptoms and infiltrations on chest radiography occurred soon after initiating treatment with valproic acid. After discontinuing valproic acid and initiating steroid therapy, the patient's symptoms and radiological findings had improved. Therefore, the patient was diagnosed with interstitial lung disease caused by valproic acid administration.
Accordingly, we propose that clinicians should be made aware of the potential for valproic acid to induce lung injury. In addition, the administration of valproic acid for the treatment of seizure disorders may require special attention.
Figures and Tables
Figure 2
At day 5 after the initiation of valproic acid, chest radiography revealed bilateral consolidation in both lower lung fields (A). A high-resolution computed tomography scan of the chest revealed predominantly dependent consolidation with air bronchogram and ground-glass opacities in both lower lobes (B).
References
1. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013; 46:1323–1338.
2. Kamenetsky Z, Da'as N, Esayag Y, Kleinman Y, Samuels N. Valproic acid-induced eosinophilic pleural effusion: a case report and review of the literature. Neurologist. 2012; 18:39–40.
3. Choi KH, Nam TS, Kim JT, Choi SM, Park MS, Kim BC, et al. Valproate associated diffuse alveolar hemorrhage. Eur J Neurol. 2011; 18:e98–e99.
4. Bullington W, Sahn SA, Judson MA. Valproic acid-induced eosinophilic pleural effusion: a case report and review of the literature. Am J Med Sci. 2007; 333:290–292.
5. Cooper JA Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 2: Noncytotoxic drugs. Am Rev Respir Dis. 1986; 133:488–505.
6. Cooper JA Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis. 1986; 133:321–340.
7. Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004; 71:301–326.
8. Shi JH, Yan XW, Xu WB, Liu HR, Zhu YJ. Clinicopathological manifestations of drug-induced lung injury. Zhonghua Jie He He Hu Xi Za Zhi. 2007; 30:161–166.
9. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012; 13:39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download