Abstract
We hereby report a case on bronchogenic cyst which is initially non-infected, then becomes infected after bronchoscopic ultrasound (US)-guided transesophageal fine-needle aspiration (FNA). The non-infected bronchogenic cyst appears to be filled with relatively echogenic materials on US, and the aspirate is a whitish jelly-like fluid. Upon contrast-enhanced MRI of the infected bronchogenic cyst, a T1-weighted image shows low signal intensity and a T2-weighted image shows high signal intensity, with no enhancements of the cyst contents, but enhancements of the thickened cystic wall. The patient then undergo video-assisted thoracic surgery 14 days after the FNA. The cystic mass is known to be completely removed, and the aspirate is yellowish and purulent. To understand the image findings that pertain to the gross appearance of the cyst contents will help to diagnose bronchogenic cysts in the future.
Bronchogenic cysts are closed epithelial-lined sacs believed to be the result of an abnormal budding process that occurs during the early development of the foregut. When this abnormal budding occurs during early gestation, the cysts tend to be located along the tracheobronchial tree, usually in the middle or posterior mediastinum (mediastinal bronchogenic cyst [MBC]). Cysts that arise later during gestation are more peripheral and may be located within the lung parenchyma itself where they often have a patent bronchial communication1,2 (bronchogenic cyst of the lung).
Chest computed tomography (CT) misclassifies these lesions as soft tissue masses in 43% of patients3. The increased attenuation observed on chest CT scans can be caused by an infection in the cyst or by high levels of proteins or calcium oxalate in the cyst content4. Magnetic resonance imaging (MRI) can improve the diagnostic accuracy by demonstrating markedly increased signal intensity within such lesions on T2-weighted image (T2WI)3. Endoscopic or bronchoscopic ultrasound (US) easily detects MBCs, which are distinguished from vascular structures by the absence of a Doppler flow signal. Endoscopic or bronchoscopic US also allows for cytological diagnosis and treatment by drainage.
Here, we report the correlation of US or MRI image with bronchogenic cyst contents. A non-infected cyst contains whitish jelly-milk-like material and the cyst appears as a homogeneous echoic lesion with fine particles on US. An infected cyst contains yellowish turbid material and appears low signal density on T1-weighted image (T1WI) and high signal density on T2WI. The serial changes in the gross appearance of the cyst content when infection develops in a bronchogenic cyst, to our knowledge, have not been reported previously.
A 51-year-old woman without a significant medical history presented to our hospital complaining of chest pain lasting 2 months. She was a non-smoker. Chest CT revealed a rounded lesion, 2.3 cm in maximum diameter, with homogeneous soft-tissue attenuation density. The lesion was located at the right lower paraesophageal space (Figure 1A). The physical examination and results of routine laboratory tests were normal. We performed bronchoscopic US (CP-EBUS, BF-UC260F-OL8; Olympus, Tokyo, Japan) via the transesophageal route to characterize the lesion. A dedicated ultrasound scanner (EU-C2000; Olympus) was used as the image processor. US depicted a 2.3-cm, round, hypoechoic lesion with some fine homogenous internal echoes and the absence of a Doppler flow signal (Figure 1B). To rule out a malignant lymphadenopathy, transesophageal fine-needle aspiration (FNA) biopsy was performed with a 22-gauge needle (Olympus NA-202C, 4 passes) under bronchoscopic US guidance. Whitish jelly-milk-like content was aspirated (Figure 1C), fluid cytology showed no evidence of malignancy and Gram staining and cultures of the mucous content showed no organisms. Fever, chest pain, and dysphagia developed within a few days thereafter, and leukocytosis was noted. Cystic infection was suspected, but to rule out other serious complications such as mediastinitis, chest MRI was performed and intravenous administration of antibiotics (ampicillin/sulbactam, 3 g q 8 hr) was begun. The cystic lesion increased in maximum diameter from 2.3 cm to 2.6 cm. Upon contrast-enhanced MRI, a T1WI showed low signal intensity, and a T2WI showed heterogeneous high signal intensity, no enhancement of the cyst contents, but enhancement of the thickened cystic wall (Figure 2). The patient underwent video-assisted thoracic surgery (VATS) 14 days after the FNA. The cystic mass was completely removed, and the aspirate was yellowish and turbid (Figure 3A). The resection specimen consisted of a benign cyst with a respiratory-type epithelial lining, smooth muscle, and epithelial denudation with infiltration of neutrophils and lymphocytes. The findings favored a diagnosis of bronchogenic cyst with active inflammation (Figure 3B, C). The MRI findings, the change in the cyst contents, and the pathology findings suggested that a secondary infection occurred after the FNA. Cultures of the aspirate were negative, probably because VATS was performed after 12 days of antibiotic therapy. The postoperative course was unremarkable. The patient's symptoms improved within a few days after the surgery, and no recurrence was seen upon follow-up chest CT at 8 months.
Bronchogenic cysts are lined by secretory respiratory epithelium. Thus, cyst fluid consists primarily of water admixed with various amounts of thick proteinaceous mucus1. Calcium oxalate crystals are sometimes detected in the fluid3. This variability in cyst content is probably responsible for the variability of attenuation seen upon CT and the signal intensity seen upon MRI. Upon chest CT, bronchogenic cysts manifest as spherical masses of either water or soft-tissue attenuation3. When a bronchogenic cyst manifests as a soft-tissue attenuation mass on a CT scan, differentiation from a solid lesion can be problematic.
Our patient presented with only compressive symptoms initially, but a cystic infection developed after transesophageal FNA (22G, for which no preventive antibiotics had been given). We could observe a change in cyst contents before and after the cystic infection. The aspirate from the non-infected cyst was whitish and jelly-milk-like, whereas the aspirate from the infected cyst appeared yellow and purulent. Chest CT showed a round lesion of homogeneous and soft-tissue attenuation density, and transesophageal US showed a smooth margin and well-circumscribed homogeneous hypoechoic cyst with fine internal echoes mimicking malignant neoplasm or adenopathy. We performed bronchoscopic US-guided transesophageal FNA and confirmed a bronchogenic cyst with thick mucinous contents, which had been depicted as soft-tissue attenuation on the CT image. Thus, we encountered a cyst complicated by infection and were able to observe the characteristic gross appearance of the mucous content of a non-infected and infected bronchogenic cyst in the same patient. Several studies have shown that mediastinal cysts can be successfully diagnosed and treated by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA)5 or endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA)6. In a prospective study conducted by Wiersema et al.7, however, EUS-FNA of cystic lesions (2 mediastinal cysts, 18 pancreatic cysts, 2 rectal cysts) was associated with a high incidence (3/22, 14%) of infectious or hemorrhagic complications (2 mediastinal cyst infections, 1 pseudocyst hemorrhage) compared with the incidence in solid lesions (2/452, 0.5%). Several case studies have documented mild to severe infective complications after EBUS-TBNA or EUS-FNA of a bronchogenic cyst7-10. Although prophylactic antibiotics for EBUS-TBNA or EUS-FNA do not appear to be indicated on the basis of risk of bacteremia, further evaluation is required to determine the role of prophylactic antibiotics in specific patient groups. Use of antibiotic prophylaxis may be appropriate in patients with relatively avascular lesions such as cysts or necrotic lymph nodes, or in immunocompromised patients at high risk of local infective complications11. Some authors have recommended that the prophylactic administration of antibiotics and a single passage of the needle be considered when aspiration of a suspected cystic lesion is to be performed9. Sato et al.5 recommended that a new needle must be used if more than one pass is necessary, and Eloubeidi et al.12 suggested that a large Tru-Cut needle (19 gauge) be avoided. In our case, no antibiotics were given, and multiple aspirations (4 passes) were performed with the same needle (22-guage). This might have increased the risk of cystic infection.
MRI can be useful in showing the cystic nature of the mass, because the cyst continue to have characteristically high signal intensity when imaged with T2-weighted sequences, regardless of the nature of the cyst contents3. Although cysts show high signal intensity on T2WI regardless of their contents, various patterns of signal intensity are seen on T1WI due to variation in the cyst contents and the presence of protein, hemorrhage, or mucoid material. In our case, in which an infectious complication developed after transesophageal aspiration, the infected cyst showed a hypointense, low signal on T1WI. This was not consistent with a previous report of an infected cyst that appeared hyperintense on T1WI13. We believe the discrepancy occurred because the T1WI signal intensity of cysts is most likely to be influenced not by infection but by the cyst contents. The high T1WI signal intensity of lesions depicted by chest MRI is thought to reflect an intracystic protein component, myxoid material, or subacute hemorrhage14. Further study is needed to clarify the discrepancy in the T1WI features of infected cysts.
There is considerable debate over the preferred therapeutic approach to mediastinal cysts. The traditional approach to mediastinal cysts, especially those with atypical radiologic features or that are symptomatic, is surgical excision for both a definitive diagnosis and treatment15. The management of asymptomatic lesions remains controversial, but most authors recommend early surgical resection. This recommendation is based on the facts that a definitive diagnosis can be obtained only by the histopathologic study of resected specimens, that most patients will become symptomatic at some point, that surgical complications most often occur when patients are symptomatic by the time of the intervention, and that there is a risk of malignant transformation.
Finally, we were able to diagnose bronchogenic cyst by transesophageal FNA using bronchoscopic US (XBF-UC 160F; Olympus). Such diagnosis has not been reported previously. The procedure is easily tolerated by patients, because the bronchoscopic US is thinner than an endoscope and easier to insert via the esophagus.
In conclusion, when clinically or radiologically indicated, endoscopic or bronchoscopic US with or without needle aspiration can be used in the diagnosis of bronchogenic cysts for differentiation of the lesion from other mediastinal processes such as lymphadenopathy or neoplasm. To prevent complications such as infection, preventive antibiotics should be considered because multiple aspirations can cause cystic infection, as shown in our case. It is imperative to minimize the number of needle aspirations in diagnosing a bronchogenic cyst that yields atypical findings upon CT or US, especially when the aspirated materials have a characteristic whitish jelly-milk-like appearance.
Figures and Tables
Figure 1
(A) Baseline computed tomography image of the chest showing a round, right lower paraesophageal lesion, of 2.3 cm in diameter, with homogeneous and soft-tissue attenuation density (arrow). (B) Transesophageal ultrasound image showing a well-circumscribed homogeneous hypoechoic lesion with fine internal echoes and a smooth margin. (C) The cyst contents appear to be whitish and jelly-milk-like.
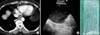
Figure 2
(A-C) Magnetic resonance images. (A) T1-weighted image (T1WI) showing low signal intensity (long arrow). (B) T2-weighted image (T2WI) showing heterogeneous high signal intensity (short arrow). (C) Under contrast enhancement, the cyst wall appears to be enhanced, but the cyst contents are not enhanced (arrowhead).

Figure 3
(A) Gross surgical specimen of the infected bronchogenic cyst with a yellowish turbid content. (B) Pathology slide showing a portion of the cyst wall with respiratory epithelium (arrow) that overlies the smooth muscle (H&E stain, ×20). (C) Epithelial denudation with infiltration of neutrophils and lymphocytes (H&E stain, ×200).

References
1. Maier HC. Bronchiogenic cysts of the mediastinum. Ann Surg. 1948; 127:476–502.
2. St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, et al. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg. 1991; 52:6–13.
3. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000; 217:441–446.
4. Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest. 1994; 106:79–85.
5. Sato M, Irisawa A, Bhutani MS, Schnadig V, Takagi T, Shibukawa G, et al. Gastric bronchogenic cyst diagnosed by endosonographically guided fine needle aspiration biopsy. J Clin Ultrasound. 2008; 36:237–239.
6. Casal RF, Jimenez CA, Mehran RJ, Eapen GA, Ost D, Sarkiss M, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010; 90:e52–e53.
7. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997; 112:1087–1095.
8. Annema JT, Veselic M, Versteegh MI, Rabe KF. Mediastinitis caused by EUS-FNA of a bronchogenic cyst. Endoscopy. 2003; 35:791–793.
9. Wildi SM, Hoda RS, Fickling W, Schmulewitz N, Varadarajulu S, Roberts SS, et al. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc. 2003; 58:362–368.
10. Hong G, Song J, Lee KJ, Jeon K, Koh WJ, Suh GY, et al. Bronchogenic cyst rupture and pneumonia after endobronchial ultrasound-guided transbronchial needle aspiration: a case report. Tuberc Respir Dis. 2013; 74:177–180.
11. Steinfort DP, Johnson DF, Irving LB. Infective complications from endobronchial ultrasound-transbronchial needle aspiration. Eur Respir J. 2009; 34:524–525.
12. Eloubeidi MA, Cohn M, Cerfolio RJ, Chhieng DC, Jhala N, Jhala D, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004; 102:253–258.
13. Jeung MY, Gasser B, Gangi A, Bogorin A, Charneau D, Wihlm JM, et al. Imaging of cystic masses of the mediastinum. Radiographics. 2002; 22 Spec No:S79–S93.
14. Barakos JA, Brown JJ, Brescia RJ, Higgins CB. High signal intensity lesions of the chest in MR imaging. J Comput Assist Tomogr. 1989; 13:797–802.
15. Sirivella S, Ford WB, Zikria EA, Miller WH, Samadani SR, Sullivan ME. Foregut cysts of the mediastinum: results in 20 consecutive surgically treated cases. J Thorac Cardiovasc Surg. 1985; 90:776–782.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download