Abstract
We hereby observe four co-infection cases of pandemic influenza H1N1 and Mycobacterium tuberculosis with various clinical presentations. It may be prudent to consider M. tuberculosis co-infections when patients with pandemic influenza reveal unusual clinical features that do not improve despite appropriate treatments against the influenza, especially in Korea, in the endemic areas of M. tuberculosis.
Pandemic H1N1 2009 influenza (pH1N1) first appeared in March 2009 in Mexico. It rapidly spread worldwide, and a pandemic was declared by the World Health Organization in June 20091. Both Mycobacterium tuberculosis (MTB) and influenza have been known to impair immune responses such as host T-cell immunologic reactions2,3. Therefore, influenza may promote the emergence of active tuberculosis in persons with latent tuberculosis infections4, and persons with tuberculosis may be vulnerable to influenza infections2,5. In addition, increased mortality by MTB has been observed during influenza pandemics6,7.
As with other pandemic influenza strains, an additive effect of MTB has been suggested with pH1N1 influenza infection, and tuberculosis was suggested as a possible risk factor associated with fatal pH1N1 influenza infection8. However, there have been few reports about co-infection of pH1N1 and MTB, and thus we describe four patients who were infected with pH1N1 as well as being confirmed with active MTB infections.
A previously healthy 20-year-old woman was admitted on December, 2009 because of abdominal pain, fever and 10 kg of weight loss during the previous two months. The physical examination upon admission revealed a high body temperature (39.3℃). Laboratory findings were as follows: white blood cells (WBC), 4,110/mm3; neutrophils, 76.4%; lymphocytes, 480/mm3 (11.7%); hemoglobin, 10.2 g/dL; C-reactive protein (CRP), 34.9 mg/L; and erythrocyte sedimentation rate, 62 mm/hr, and a stool occult blood test was positive. A chest X-ray showed granular opacity in the bilateral apical area. Repetitive sputum acid fast bacilli (AFB) fluorescent smear studies were negative. A computed tomographic (CT) scan of the abdomen and pelvis revealed diffuse swelling of the liver, gallbladder, distal small bowel and ascending colon. Considering the influenza pandemic period and opacities in the chest X-ray, a reverse transcriptase polymerase chain reaction (RT-PCR) for pH1N1 via nasopharyngeal swab specimen was performed, and pH1N1 infection was confirmed on hospital day (HOD) 2. The conventional dose of oral oseltamivir (75 mg b.i.d.) was prescribed to the patient. On the same day, the patient complained of hematochezia, and then sigmoidoscopic evaluation revealed several polypous and aphthous lesions on the cecum and rectum with erythema, edema, granularity and telangiectasia, while pathologic evaluation of the biopsy specimen revealed acute colitis. Fever and respiratory symptom improved after three days of treatment with oseltamivir, but hematochezia had been continued, and after 5 afebrile days, fever started to spike again with higher degree up to 39.7℃. An interferon-gamma releasing assay (QuantiFERONTB Gold; Cellestis Ltd., Carnegie, Australia) was positive, and the pathology result of colonoscopy done for further evaluation of hematochezia revealed a chronic granulomatous inflammation consistent with intestinal tuberculosis, and a Ziehl-Neelsen stain revealed acid-fast bacilli. On the next day of pathology report, a MTB complex-specific PCR of sputum revealed positive results, and the administration of isoniazid, rifampin, ethambutol and pyrazinamide was initiated (HOD 16). Symptoms of fever, abdominal pain and hematochezia gradually improved within 5 days of treatment with anti-tuberculosis mediation, and the growth of MTB was confirmed in the sputum specimens after three weeks of collection.
A previously healthy 47-year-old man was referred to the emergency department (ED) on December, 2009 for dyspnea and documentation of pneumonic consolidation in chest X-rays from another primary care clinic. He complained of two weeks of cough, sputum, and rhinorrhea. Dyspnea upon exertion had developed since the day before he visited the ED. Laboratory findings were as follows: WBC, 8,260/mm3; neutrophils, 80.2%; lymphocytes, 1,050/mm3 (11.7%); and CRP, 79.6 mg/L. His chest X-ray image revealed heterogenous ground glass opacities in bilateral lung fields. For further evaluation of the pulmonary lesions, a chest CT was performed, and multisegmental involvement of centrilobular nodules with a tree-in-bud appearance and multiple small cavitary lesions were observed, suggesting active pulmonary tuberculosis. In addition, RT-PCR for pH1N1 via nasopharyngeal swab was positive, and a serum glucose test registered 404 mg/dL. Admission was recommended for proper management of pH1N1 and active pulmonary tuberculosis as well as previously unknown diabetes mellitus. However, the patient refused admission and was discharged with medications such as oseltamivir, isoniazid, rifampin, ethambutol, and pyrazinamide. An AFB smear reported to be 4+ next day, and improvement of symptoms and pulmonary consolidation in chest X-ray were observed upon out-patient department follow-up visit after 5 days. Later, growth of MTB was confirmed in respiratory specimens. It took about 6 months for the lung lesions of heterogenous ground glass opacities and consolidations to nearly resolve.
A 45-year-old man visited the ED on December, 2009 because of fever and cough for one day and one incident of hemoptysis estimated at about 400 mL. He had a history of pulmonary tuberculosis diagnosed in 2007 and reported a complete recovery after treatment. A physical examination upon admission revealed a high body temperature (38.1℃) and increased pulse rate (105/min), while his breath sounds were clear. Oxygen saturation was 97% under an oxygen supply of 2 L/min via nasal cannula. Laboratory findings were as follows: WBC, 7,110/mm3; neutrophils, 80.8%; lymphocytes, 650/mm3 (9.1%); and CRP, 6.69 mg/L. A chest X-ray showed multiple small nodules and patchy consolidation predominantly in the left lung field, and chest CT revealed a cavitary nodule and tree-in-bud appearance on the left upper lung and left lingular segment, with ground glass opacities due to aspirated blood on the left lower lung suggesting tuberculosis reactivation (Figure 1). However, pH1N1 RT-PCR was positive for a nasopharyngeal swab specimen, and a conventional dose of oral oseltamivir was prescribed. Fever spiking was improved after starting oseltamivir, but it remained to lower degree for 4 days. Repetitive sputum AFB smears were negative, while MTB complex-specific PCR was positive. Due to suspected active pulmonary tuberculosis, isoniazid, rifampin, ethambutol and pyrazinamide were prescribed on HOD 4. Fever subsided from the next day of starting anti-tuberculosis medication, and respiratory symptoms gradually improved, so the patient was discharged on HOD 6. Three weeks after collection, a sputum culture revealed growth of MTB.
An 88-year-old woman was transferred to the ED on January, 2010 for suspected panperitonitis. She had been diagnosed with pneumonia during a prior hospital admission and reported that related symptoms improved after 14 days of intravenous ceftriaxone and metronidazole administration. Upon arrival, the patient complained abdominal pain, mild dyspnea, and sputum. The physical examination revealed no significant findings except for diffuse tenderness in her whole abdomen. Laboratory findings were as follows: WBC, 12,810/mm3; neutrophils, 89.2%; lymphocytes, 760/mm3 (6.0%); and CRP, 182 mg/L. Chest X-ray revealed a mass-like lesion in the left upper lung field, and subsequent chest CT revealed left bronchial wall narrowing and obstruction with distal consolidation. However, pH1N1 RT-PCR revealed a positive result in a nasopharyngeal specimen, and a conventional dose of oseltamivir was administered as along with piperacillin/tazobactam. The patient was thought to also have pseudomembranous colitis according to clinical presentation and abdomen CT finding, and abdominal pain was improved after administration of oral vancomycin.
Despite administration of both anti-bacterial and anti-viral agents, pulmonary consolidation did not improve while dyspnea and sputum had been improving. After repetitive negative results of sputum AFB smear studies, growth of MTB was identified from the initial respiratory specimens on HOD 15. After administration of isoniazid, rifampin, ethambutol, and pyrazinamide, pulmonary infiltration observed via chest X-ray improved, and the patient was discharged without significant sequelae.
Interaction between influenza A virus and pulmonary tuberculosis has been suggested, and underlying tuberculosis infection is considered one of the identifiable risk factors for severe influenza infection2,5. This is not only because of the immunosuppression in tuberculosis, but also because significant percentages of patients treated for tuberculosis are left with sequelae of the disease including bronchiectasis, which may predispose them to life-threatening influenza A infections6.
There have been a few reports of fatal co-infection cases of pandemic influenza and active tuberculosis in South Africa6,8. However, South Africa is one of the countries with the highest burden of tuberculosis (993 cases per 100,000 population)9, and the clinical presentations of tuberculosis are not identifiable in these reports. Our data is one of the rare cases of co-infection of pandemic influenza and active pulmonary tuberculosis in an intermediate tuberculosis-burden country (100 cases per 100,000 population)9. We described four cases of MTB and pH1N1 co-infection and the variations in their clinical presentations. The diagnosis of tuberculosis was delayed in case no. 1 because the chief complaints were gastrointestinal symptoms and repeated sputum AFB smear studies were negative, although the patient proved to have been an active case of pulmonary tuberculosis. In addition, it was not until the confirmation of tuberculosis through colonoscopic biopsy pathology that the positive result of sputum MTB PCR was available. In fact, the symptoms of abdominal pain and weight loss for 2 months, and chest X-ray finding of granular opacity in both apical lung fields instead of lower lung fields do not seem typical for H1N1 infection but instead more close to tuberculosis, when retrospectively seen. But positivity for pH1N1 and initial improvement of fever and respiratory symptom after starting oseltamivir might preclude us from early suspicion of active tuberculosis. In cases nos. 2 and 3, the patients was suspected to have an active pulmonary tuberculosis infection, considering past history of pulmonary tuberculosis and typical clinical features of hemoptysis in case no. 3 and cavitary nodules in chest CT study in cases nos. 2 and 3. However, in case no. 3, repeated AFB smear studies were negative despite the classical features. Fortunately, MTB PCR was positive in an initial study, and treatment for tuberculosis was initiated relatively early in this case. In contrast, the diagnosis of tuberculosis was delayed in case no. 4. She was considered as a bacterial pneumonia case combined with pH1N1 infection. Repeated sputum AFB studies were negative, and MTB PCR was not performed, unfortunately. But considering that obstructive pneumonia is rare in H1N1 infection, tuberculosis could have been suspected.
Noh et al.10 previously reported 7 cases of concurrent MTB and influenza infection in South Korea. They were mostly previous healthy, and clinical outcomes were good for all patients. Among 4 patients with available initial lymphocyte count, 2 patients were lymphopenic. In our case reports, three among the four patients had prior medical co-morbidities such as prior pulmonary tuberculosis, bacterial pneumonia, and diabetes mellitus, and the clinical presentations of the co-infection cases of MTB and the pH1N1 influenza were diverse. But these patients have some characteristics in common. The clinical presentation and image findings of all patients were not typical for H1N1 infection, but rather closer to MTB infection. But the diagnosis of pH1N1 was made in advance, and this may preclude or mask the diagnosis of tuberculosis in these patients. Considering these, it seems more likely that the patients who had tuberculosis went through a transient or incidental infection of pH1N1 which might have aggravated patients' symptoms. Whether these patients were more susceptible to pH1N1 because of having tuberculosis infection is not clear. One study reported that pulmonary MTB was a risk factor for pH1N1 infection5. However, the reports about the co-infection of MTB and influenza have been mostly descriptive and could not show the causal relationship. Also, it is not clear whether influenza accelerate emergence of MTB. But animal models with mouse suggest the possibility of influenza-associated MTB11,12, and temporary suppression of T-cell immunity by pH1N1 virus was reported13, which might alter the course of MTB infection. In our cases, all 4 patients showed lymphopenia at initial visit which was recovered within 1 month. To clarify whether the rapid lymphopenia associated with pH1N1 infection is related to the host susceptibility to MTB, further studies of serial quantification and functional assay of lymphocytes at the acute stage of influenza infection and its effect are needed.
The co-infection of MTB and pH1N1 could be an incidental finding, and the possible interaction between MTB and pH1N1 is difficult to prove. Therefore, large-scale epidemiological data may be necessary to evaluate and further understand the interaction between MTB and pH1N1 infection.
Figures and Tables
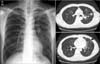 | Figure 1Chest X-ray and chest computed tomography (CT) in case no. 3. Chest X-ray revealed multiple small nodules and patchy consolidations predominantly in the left lung field, and the chest CT revealed a cavitary nodule (thin arrow) and tree-in-bud appearance in the left upper lung and left lingular segment, with ground glass opacities (thick arrow) due to aspirated blood in the left lower lung, that suggests tuberculosis reactivation. |
References
1. Centers for Disease Control and Prevention (CDC). Outbreak of swine-origin influenza A (H1N1) virus infection: Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:467–470.
2. Ellner JJ. Immunosuppression in tuberculosis. Infect Agents Dis. 1996; 5:62–72.
3. Kantzler GB, Lauteria SF, Cusumano CL, Lee JD, Ganguly R, Waldman RH. Immunosuppression during influenza virus infection. Infect Immun. 1974; 10:996–1002.
4. Lofgren S, Callans A. Asian influenza and pulmonary tuberculosis. Acta Med Scand. 1959; 164:523–527.
5. Puvanalingam A, Rajendiran C, Sivasubramanian K, Ragunanthanan S, Suresh S, Gopalakrishnan S. Case series study of the clinical profile of H1N1 swine flu influenza. J Assoc Physicians India. 2011; 59:14–16. 18
6. Koegelenberg CF, Irusen EM, Cooper R, Diacon AH, Taljaard JJ, Mowlana A, et al. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010; 103:319–325.
7. Housworth J, Langmuir AD. Excess mortality from epidemic influenza, 1957-1966. Am J Epidemiol. 1974; 100:40–48.
8. Archer B, Cohen C, Naidoo D, Thomas J, Makunga C, Blumberg L, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill. 2009; 14:pii: 19369.
9. World Health Organization. Global tuberculosis report 2012 [Internet]. Geneva: World Health Organization;2012. cited 2013 Oct 1. Available from: http://www.who.int/tb/publications/global_report/en/.
10. Noh JY, Lee J, Choi WS, Song JY, Seo YB, Kim IS, et al. Concurrent tuberculosis and influenza, South Korea. Emerg Infect Dis. 2013; 19:165–167.
11. Volkert M, Pierce C, Horsfall FL, Dubos RJ. The enhancing effect of concurrent infection with pneumotropic viruses on pulmonary tuberculosis in mice. J Exp Med. 1947; 86:203–214.
12. Co DO, Hogan LH, Karman J, Heninger E, Vang S, Wells K, et al. Interactions between T cells responding to concurrent mycobacterial and influenza infections. J Immunol. 2006; 177:8456–8465.
13. Jiang TJ, Zhang JY, Li WG, Xie YX, Zhang XW, Wang Y, et al. Preferential loss of Th17 cells is associated with CD4 T cell activation in patients with 2009 pandemic H1N1 swine-origin influenza A infection. Clin Immunol. 2010; 137:303–310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download