Abstract
Non-small cell lung cancer harboring epidermal growth factor receptor (EGFR) sensitizing mutations has a distinct disease entity. Patients with this cancer have better prognosis, and frequently achieve long-term survival. EGFR-tyrosine kinase inhibitor (TKI) is the drug of choice for this cancer; but the disease inevitably progresses, after durable response. The tumor is a mixture of EGFR-TKI sensitive clones and resistant clones, regardless of their molecular mechanisms. EGFR-TKI sensitive clones are very susceptible to this drug, but rarely eradicated; so, withdrawal of the drug permits rapid regrowth of drug sensitive clones, possibly causing "disease flare." Re-administration or continuation of EGFR-TKI can effectively suppress the expansion of drug sensitive clones, even when the total tumor volume continuously increases. Chemotherapy can definitely prolong the survival of patients experiencing EGFR-TKI failure. Prospective clinical trials are warranted to compare efficacies of chemotherapeutic agents. A few retrospective studies suggested that a taxane-based regimen may be superior to others. Here, we reviewed therapeutic options and clinical evidence about this unique disease entity.
Lung cancer is a leading cause of cancer mortality in the world, and also was ranked as the 4th incidence following thyroid, stomach, and colon carcinoma according to year 2011 cancer statistics of the Korea central cancer registry. Mutation of epidermal growth factor receptor (EGFR) is a major driver oncogene of non-small cell lung cancer (NSCLC), especially adenocarcinoma and it is more frequently discovered in patients with clinical factors of female, never or light smoker, adenocarcinoma, and Asian ethnicity. Korean Cardio-pulmonary Pathology Study Group conducted a Korea nationwide survey about EGFR mutation status of NSCLC diagnosed in year 2009 (n=1,753 from 15 hospitals), which was demonstrating that EGFR mutation was discovered in 43.3% of adenocarcinoma, 8.3% of large cell carcinoma, and 8.9% of squamous cell carcinoma. It was discovered in 48.1% of non-smoker, 43.6% of <10 pack-year light smoker, 15.9% of ex-smoker and 19.8% of current smoker. Fifty point three percent of female and 22.3% of male patients had EGFR mutation1. This survey strongly suggests that EGFR mutation should be tested in all patients with lung adenocarcinoma because substantial portion of smoker and male patients have EGFR mutation in Korea, which is different to western society.
Lung adenocarcinoma harboring sensitizing EGFR mutations very frequently responds EGFR-tyrosine kinase inhibitor (TKI). EGFR mutations are categorized into two groups, EGFR-TKI sensitizing (or activating) mutations (mostly exon 19 deletions, L858R or G719X substitution) vs. resistant mutations (mostly T790M or insertion on exon 20). The frequency of sensitizing vs. resistant mutations is reported as about 95% vs. 5%2. The impact of EGFR mutation on chemotherapy outcomes is still a matter of debate, but several studies have reported that chemotherapeutic response rates and/or survival outcomes are also better in patients with EGFR mutations than those with wild type EGFR3-7. Consequently, better outcomes with EGFR-TKI and chemotherapy are projected on overwhelmingly better survival of patients with EGFR sensitizing mutations than those without the mutation. The European Randomized Trial of Tarceva versus Chemotherapy (EURTAC), a clinical trial with erlotinib as the fist line treatment for stage IV European patients with EGFR sensitizing mutations demonstrated 19.3 months of median overall survival (OS)8. First line gefitinib studies for East Asian patients such as IRESSA Combined Analysis of the Mutation Positives (I-CAMP), IPASS, NEJ002 and WJTOG3405 also demonstrated much better median OSs (22-36 months) compared with generally known median survival data (11-13 months) of advanced staged NSCLC4,9-13. Clinical research data have suggested around 35% of 3-year survival rate, 20% of 4-year survival rate, and 10% of 5-year survival rate of patients with advanced stage NSCLC harboring EGFR-TKI sensitizing mutations. Therefore, we should have a clear understanding and therapeutic plans for these long term survivors.
Clinical trials have consistently proved that the first line EGFR-TKI treatment showed better outcome in terms of progression free survival (PFS) compared with cytotoxic chemotherapy for patients with NSCLC harboring EGFR sensitizing mutations. All the clinical trials with the first line gefitinib, I-CAMP (non-randomized pooled analysis), IPASS (sub-set analyses of the phase III randomized clinical trials), NEJ002, WJTOG3405 (phase III of randomized clinical trials for mutant EGFR NSCLC patients) demonstrated significantly better PFS, 0.30-0.61 of hazard ratio (HR) for progression, compared with chemotherapy4,9,11,13. Clinical trials with erlotinib, OPTIMAL, EURTAC (phase III randomized clinical trials for mutant EGFR NSCLC patients) also proved better PFS, 0.16-0.37 of HR for progression, compared with chemotherapy8,14. However, every clinical trial consistently has failed to prove the benefit of OS over chemotherapy because of post-study heavy cross-over treatment between the trial arms. This suggests EGFR-TKI will give patients survival benefit at any treatment line. But the most effective anti-cancer agent should be tried first based on two important points. The one is if a patient is suddenly deteriorated during less effective treatment, the patient may lose a chance to be treated with the most effective drug and die. The other is that quality of life will be maximally improved with the most effective treatment. Although drugs have their specific toxicity profiles, target agents have milder toxicity profiles than cytotoxic agents in general. According to IPASS study, the patients given gefitinib showed better quality of life than those given cytotoxic chemotherapy in NSCLC with activating EGFR mutations. On the contrary, the patients given cytotoxic chemotherapy showed better quality of life than those given gefitinib in NSCLC with wild type EGFR15. This suggests efficacy of drug is more important factor to determine quality of life than drug toxicity profile. Currently, National Comprehensive Cancer Network (NCCN)'s and several others' treatment guidelines recommend EGFR-TKI as the first line treatment for patients with NSCLC harboring EGFR sensitizing mutations (Figure 1)16.
NCCN guideline recommends adding EGFR-TKI to chemotherapy or EGFR-TKI maintenance after 4 cycles of chemotherapy if the presence of activating EGFR mutations was noticed after starting cytotoxic chemothrapy16. There are two randomized clinical trials testing efficacy of erlotinib (Sequential Tarceva in Unrectable NSCLC [SATURN]) or gefitinib (INFORM) as switch maintenance17,18. Both of the trials were conducted for patients with stage IIIB-IV NSCLC showing non-progressive response after 4 cycles of platinum based doublet chemotherapy. EGFR status was not a condition of eligibility in both trials. SATURN trial (n=889, Caucasian comprising 84% of study population) showed significant PFS benefit (0.72 of HR for progression; 95% confidence interval [CI], 0.62-0.82) and also OS benefit (0.81 of HR for death; 95% CI, 0.70-0.95) in patients given erlotinib maintenance compared with placebo. INFORM trial (n=296, Chinese comprising 100% of study population) demonstrated only PFS benefit (0.42 of HR for progression; 95% CI, 0.33-0.55) but failed OS benefit (0.84 of HR for death; 95% CI, 0.62-1.14) in patients given gefitinib maintenance compared with placebo. The difference in OS benefit of two trials may be caused by drugs, ethnicity, or study population scale of the trials. Subgroup analyses for patients with activating EGFR mutations proved PFS benefits in both trials (0.10 of HR for progression in SATURN trial; 95% CI, 0.04-0.25 and 0.17 of HR for progression in INFORM trial; 95% CI, 0.07-0.42) but failed to prove OS benefit.
The median PFS of EGFR-TKI as the first line chemotherapy ranged about from 10 to 14 months in patients with NSCLC harboring activating EGFR mutations. Cytotoxic chemotherapy can provide survival benefit to patients experiencing EGFR-TKI failure. NCCN guideline recommends docetaxel, pemetrexed or platinum doublet chemotherapy±bevacizumab as the second-line therapy. This recommendation is backed by clinical evidences based on the trials for patients with advanced NSCLC regardless of EGFR status, but not on trials targeting patients with EGFR mutated NSCLC. Thus, it is not clear whether the same guideline can be applied to patients with this unique disease entity.
A retrospective study compared taxane based and gemcitabine based regimens as the first-line chemotherapy in patients with advanced NSCLC. Response rate, disease control rate, and PFS were not different between two regimens in patients with wild type EGFR. On the contrary, disease control rate and PFS of taxane based regimen was superior to gemcitabine based regimen in patients with EGFR sensitizing mutations19. Another retrospective study demonstrated salvage chemotherapy provided survival gain to patients with advanced NSCLC when disease progressed after durable disease control with EGFR-TKI. Median PFS of patients given salvage chemotherapy was superior to that of supportive care group (3.5 months vs. 1.5 months, p<0.01), and median survival from the date of EGFR-TKI cessation to death was also superior in the salvage chemotherapy group (11.2 months vs. 3.8 months, p<0.01). Intriguingly, patients given taxane based regimen showed better PFS than those given non-taxane based regimen (5.1 months vs. 1.8 months, p<0.01) and also better median survival from the date of EGFR-TKI cessation to death (12.7 months vs. 7.0 months, p<0.01)20. These two studies suggest taxane based regimen may be superior to non-taxane based regimen for NSCLC harboring EGFR sensitizing mutations. Prospective randomized clinical trials are warranted to figure out efficacy of each chemotherapy agent for this disease.
It is very important that cancer cells with EGFR sensitizing mutations have significant sensitivity to a specific chemotherapeutic agent. Chemotherapy can rarely induce complete remission of metastatic NSCLC. Therefore, if a specific anti-cancer agent can delay progression as far as possible, it can be directly associated with a patient's prognosis. If salvage chemotherapy after EGFR-TKI failure comprise of platinum based doublet like as the first line chemotherapy, it is likely to be given at most 6 cycles because of drug toxicities. However, cancer cells especially sensitive to a specific drug, this agent as a monotherapy can suppress tumor growth more over 6 cycles, which is translating into a patient's survival gain. Cancer cells achieving EGFR-TKI resistance will not respond to EGFR-TKI anymore for a certain period, therefore they should be controlled by cytotoxic chemotherapy. However, it is unclear whether cytotoxic chemotherapy should be combined with EGFR-TKI (continuation of EGFR-TKI) or not (transient withdrawal of EGFR-TKI). Randomized clinical trials are warranted to find out the best option for these clinical situations.
Patients treated with EGFR-TKI inevitably experience acquired resistance by various molecular mechanisms such as T790M mutation on EGFR exon 20, activation of alternative pathways (c-MET, IGF-1, AXL, etc.), small cell transformation, and tumor heterogeneity. If possible, rebiopsy is recommended because it will inform a physician of underlying mechanism of resistance which may be successfully managed by another treatment option. T790M mutation is the most common mechanism comprising about 50% of all resistant cases. It is unclear that T790M mutation is a secondary gain by cancer cells to survive from lethal drug exposure or a result of selection by drug among mixture of cells with EGFR-TKI sensitizing mutation or T790M mutation initially undetected because of very small fraction. Each resistant cancer cell may have one or more mechanisms simultaneously. Clinically, tumor mass is comprising of EGFR-TKI sensitive clones and resistant clones regardless of their molecular mechanisms. EGFR-TKI decreases the population of drug sensitive clones but rarely eradicates them. On the other hand, the population of EGFR-TKI resistant clones continuously grows despite of EGFR-TKI treatment. Although tumor rapidly shrinks for some time by EGFR-TKI, growth of drug resistant cell mass will mask the effect, eventually showing "disease progression" or "EGFR-TKI failure." If EGFR-TKI is withdrawn at disease progression, EGFR-TKI sensitive clones will rapidly regrow and possibly cause "disease flare" phenomenon (Figure 2)21. Tumor doubling time will be determined by each doubling time of EGFR-TKI sensitive clones and resistant clones, mixed ratio of the clones, and response to chemotherapy if it is given.
Regrowing EGFR-TKI clones will also respond to EGFR-TKI retreatment. Theoretically retreatment efficacy may be affected by drug holiday period to allow mass forming of EGFR-TKI sensitive clones. A retrospective study showed that 40% (2/5) of patients with both EGFR exon 19 deletion mutation and EGFR T790M mutation on rebiopsy done at EGFR-TKI failure responded to retreatment after drug holiday (1/5 of them showing stable disease and 2/5 progressive disease)22. Based on experimental evidences, partial remission could not be achievable if each cancer cell was harboring dual mutations (EGFR sensitizing mutations and T790M)23,24. However, the presence of partial responder to EGFR-TKI retreatment strongly suggests the tumor was a mixture of EGFR-TKI resistant clones and rapidly growing sensitive clones after stopping the drug. In general, EGFR-TKI retreatment will give remarkably shorter PFS compared with previous treatment mainly because of steadily growing resistant clones (0-86% of disease control rate, 1.7-6.5 months of PFS22,25-27).
A prospective study assessed efficacy of discontinuation and re-initiation of EGFR-TKI in patients with acquired resistance to the drug. The eligible 10 patients had stage IV NSCLC and were treated with gefitinib or erlotinib monotherapy for more than 6 months. They had achieved radiographic response before EGFR-TKI failure or documentation of either EGFR exon 19 deletion or an EGFR L858R mutation. They stopped taking EGFR-TKI at progression for 3 weeks and were retreated with EGFR-TKI for 3 weeks. Chest computed tomography (CT) and positron emission tomography-CT was taken just before and after stopping and retreating. Three weeks after stopping EGFR-TKI, there was a median 18% increase in SUVmax and 9% increase in tumor diameter. Three weeks after restarting EGFR-TKI, there was a median 4% decrease in SUVmax and 1% decrease in tumor diameter28. This proves retreatment of EGFR-TKI can effectively inhibit tumor growth caused by withdrawal of the drug. Disease flare defined as rapid deterioration of patients' condition possibly causing death after stopping of EGFR-TKI should be a definite indication of immediate retreatment of EGFR-TKI29.
One of major revisions of year 2013 NCCN guideline for NSCLC is recommendation of continuing EGFR-TKI at drug failure for NSCLC harboring EGFR sensitizing mutations16. EGFR-TKI is indicated not only for asymptomatic progression but for symptomatic local progression. Local progressive lesion should be treated by local modality. Symptomatic multiple sites progression should be treated with chemotherapy±continuing EGFR-TKI. Even if EGFR-TKI is withdrawn, it can be re-administered after some drug holiday (Figure 1).
A retrospective study classified EGFR-TKI failure as three clinical modes; dramatic progression, gradual progression and local progression. Continuing EGFR-TKI was superior in terms of median OS to switching chemotherapy in a subsequent setting for gradual progression. Chemotherapy seemed to be better than EGFR-TKI continuation in dramatic progression. The outcomes of switching chemotherapy and continuing EGFR-TKI were similar in local progression30.
Incidence of brain metastasis in patients with NSCLC was reported to reach 25-30% until the end of lives. A study showed that the incidence was higher in patients with mutant EGFR than those with wild type EGFR (64% vs. 31%)31. It is unclear whether cancer cell with mutant EGFR has more potential to metastasize than that with wild type EGFR or patients with mutant EGFR live longer, so they have more chances of brain metastases. Symptomatic one or two metastatic lesions can be surgically resected. Metastases less than 4 and smaller than 3 cm diameter can also be controlled by stereotatic radiosurgery and/or whole brain radiotherapy. Diffuse symptomatic metastases need whole brain radiotherapy. Asymptomatic metastases of EGFR mutated NSCLC could be monitored without immediate local control because 82-89% of response rate for intracranial lesion is expected by EGFR-TKI31-33. Despite the initial good response, metastatic lesions will eventually progress because effective tumor growth inhibitory drug concentration rises over time and almost all target agents insufficiently cross blood-brain barrier, so reach under effective concentration.
Jackman et al.34 reported a case with local failure in central nervous system (CNS) controlled by high dose gefitinib. The patient with NSCLC harboring EGFR exon 19 deletion responded to gefitinib at the usual dose of 250 mg/day. However, he experienced symptomatic local progression in CNS after durable response and the CNS lesion was successfully but transiently controlled by gefitinib at doses escalating from 500 mg/day to 1,250 mg/day. Several similar cases were also reported in patients treated with erlotinib. Patients' neurologic symptoms were transiently controlled with high dose erlotinib on alternating days at a dose of 300 mg/day or 1,000-1,500 mg weekly pulse therapy35-38. Insufficient CNS drug concentration should be considered as a major cause of CNS progression if systemic lesions are still being controlled with EGFR-TKI.
Effective and minimally toxic new drugs should be continuously developed. Patients with this unique disease entity frequently survive longer than three years with still good performance. However, chemotherapeutic agents are likely no longer available at this time because all the effective drugs for NSCLC have been used once within 2-3 years after diagnosis. If patients are not candidates for clinical trials for new drugs, cytotoxic chemotherapeutic agents may also be considered as retreatment like as EGFR-TKI retreatment. To dates, cytotoxic drug retreatment has not been studied in NSCLC, but there are several retrospective studies reporting successful retreatment after a certain drug holiday in several malignancies such as leukemia, multiple myeloma, breast cancer, small cell lung cancer, ovarian cancer, etc.39.
Several strategies for overcoming resistances are under investigations. Irreversible EGFR-TKI such as afatinib and dacomitinib are on the front lines of clinical trials although unsatisfied outcomes until these days. Afatinib was recently approved by the US Food and Drug Administration (FDA) for advanced NSCLC harboring EGFR mutations. Developing novel mutant-selective EGFR-TKI, combination of target agents, or combination of EGFR-TKI and EGFR depleting agents are on the ways to overcome resistances21.
NSCLC harboring activating EGFR mutations has a distinct disease entity. Patients with this cancer have better prognosis and frequently achieve long term survival. EGFR-TKI is the drug of choice but the disease inevitably progress after durable response. Tumor is a mixture of EGFR-TKI sensitive clones and resistant clones regardless of their molecular mechanisms. EGFR-TKI sensitive clones are very susceptible to the drug, but rarely eradicated, so withdrawal of the drug causes rapid regrowth of drug sensitive clones. Re-administration or continuation of EGFR-TKI can effectively suppress the expansion of drug sensitive clones. Chemotherapy can definitely prolong survivals of patients experiencing EGFR-TKI failure. Prospective clinical trials are warranted to compare efficacies of chemotherapeutic agents. A few retrospective studies suggested that taxane based regimen may be superior to others. Although effective new drugs should be developed, clinical trial about chemotherapy retreatment will also be valuable and interesting. Brain metastasis is a frequent complication for long term survivors. Dose escalation of EGFR-TKI is an option for local CNS failure even though short responsive period. Investigations overcoming EGFR-TKI resistance should be continued.
Figures and Tables
 | Figure 1National Comprehensive Cancer Network (NCCN) practice guideline for epidermal growth factor receptor (EGFR) mutation positive non-small cell lung cancer16. TKI: tyrosine kinase inhibitor; RT, radiotherapy. |
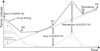 | Figure 2Schematic tumor volume-time curve according to on-and-off of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI). The tumor volume is the sum of EGFR-TKI sensitive clones and resistant clones, regardless of baseline molecular mechanisms. The tumor volume of EGFR-TKI sensitive clones rapidly shrinks with EGFR-TKI administration, but the tumor volume of EGFR-TKI resistant clones steadily increases, despite the treatment. The total tumor volume initially reaches the level of partial response (PR), but re-increases, and after a certain period, eventually progresses (progressive disease [PD]), because of expanding EGFR-TKI resistant clones. Withdrawing EGFR-TKI induces regrowth of EGFR-TKI sensitive clones, possibly causing disease flare. If EGFR-TKI is re-administered, the tumor volume can be stabilized (decreasing stable disease [SD] or PR). However, the progression free survival would be shorter than that of the initial treatment21. |
References
1. The Cardiopulmonary Pathology Study Group of Korean Society of Pathologists. Article No. 3824. Epidermal growth factor receptor mutation analysis of non-small cell lung cancer in Korea: Summary from a nationwide survey. In : 2010 Autumn Conference of the Korean Society of Pathologists; Seoul: The Cardiopulmonary Pathology Study Group of Korean Society of Pathologists;2010.
2. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007; 7:169–181.
3. Hotta K, Kiura K, Toyooka S, Takigawa N, Soh J, Fujiwara Y, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2007; 2:632–637.
4. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957.
5. Kalikaki A, Koutsopoulos A, Hatzidaki D, Trypaki M, Kontopodis E, Stathopoulos E, et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer. 2010; 69:110–115.
6. Lin CC, Hsu HH, Sun CT, Shih JY, Lin ZZ, Yu CJ, et al. Chemotherapy response in East Asian non-small cell lung cancer patients harboring wild-type or activating mutation of epidermal growth factor receptors. J Thorac Oncol. 2010; 5:1424–1429.
7. Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012; 30:3077–3083.
8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246.
9. Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, et al. Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2009; 15:4493–4498.
10. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011; 29:2866–2874.
11. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388.
12. Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013; 24:54–59.
13. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128.
14. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.
15. Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol. 2011; 6:1872–1880.
16. Clinical practice guideline in oncology: non-small cell lung cancer version 2.2013. Fort Washington: National Comprehensive Cancer Network;2013. cited 2013 Jul 27. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
17. Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010; 11:521–529.
18. Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012; 13:466–475.
19. Park JH, Lee SH, Keam B, Kim TM, Kim DW, Yang SC, et al. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer. 2012; 77:433–437.
20. Kuo CH, Lin SM, Lee KY, Chung FT, Hsieh MH, Fang YF, et al. Subsequent chemotherapy improves survival outcome in advanced non-small-cell lung cancer with acquired tyrosine kinase inhibitor resistance. Clin Lung Cancer. 2010; 11:51–56.
21. Lee JC, Jang SH, Lee KY, Kim YC. Treatment of non-small cell lung carcinoma after failure of epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Res Treat. 2013; 45:79–85.
22. Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, et al. Retreatment with erlotinib: regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011; 47:2603–2606.
23. Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011; 3:90ra59.
24. Yamada T, Matsumoto K, Wang W, Li Q, Nishioka Y, Sekido Y, et al. Hepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancer. Clin Cancer Res. 2010; 16:174–183.
25. Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer. 2012; 77:121–127.
26. Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007; 7:51.
27. Asahina H, Oizumi S, Inoue A, Kinoshita I, Ishida T, Fujita Y, et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology. 2010; 79:423–429.
28. Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007; 13:5150–5155.
29. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011; 17:6298–6303.
30. Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 2013; 79:33–39.
31. Li Z, Lu J, Zhao Y, Guo H. The retrospective analysis of the frequency of EGFR mutations and efficacy of gefitinib in NSCLC patients with brain metastases. J Clin Oncol. 2011; 29:15 Suppl. e18065.
32. Porta R, Sanchez-Torres JM, Paz-Ares L, Massuti B, Reguart N, Mayo C, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011; 37:624–631.
33. Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012; 77:556–560.
34. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006; 24:4517–4520.
35. Hata A, Kaji R, Fujita S, Katakami N. High-dose erlotinib for refractory brain metastases in a patient with relapsed non-small cell lung cancer. J Thorac Oncol. 2011; 6:653–654.
36. Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010; 99:283–286.
37. Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011; 13:1364–1369.
38. Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases: one with a remarkable thoracic response as well. Lung Cancer. 2013; 80:102–105.
39. Cara S, Tannock IF. Retreatment of patients with the same chemotherapy: implications for clinical mechanisms of drug resistance. Ann Oncol. 2001; 12:23–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download