Abstract
Immunoglobulin G4 (IgG4)-related disease is a newly recognized condition characterized by fibroinflammatory lesions with dense lymphoplasmacytic infiltration, storiform-type fibrosis and obliterative phlebitis. The pathogenesis is not fully understood but multiple immune-mediated mechanisms are believed to contribute. This rare disease can involve various organs and pleural involvement is even rarer. We report a case of IgG4-related disease involving pleura. A 66-year-old man presented with cough and sputum production for a week. Chest radiography revealed consolidation and a pleural mass at right hemithorax. Treatment with antibiotics resolved the consolidation and respiratory symptoms disappeared, but the pleural mass was unchanged. Video-assisted thoracoscopic surgery was performed. Histopathology revealed dense lymphoplasmacytic infiltration and storiform fibrosis with numerous IgG4-bearing plasma cells. The serum IgG4 level was also elevated. Further examination ruled out the involvement of any other organ. The patient was discharged without further treatment and there is no evidence of recurrence to date.
Immunoglobulin G4 (IgG4)-related disease was first described as a subtype of autoimmune pancreatitis with the characteristic findings of dense lymphoplasmacytic infiltration, storiform fibrosis with numerous IgG4 positive cells on pathology and, on occasion, elevated serum IgG4 levels1. Similar findings have been described in other diseases including sclerosing cholangitis, sclerosing sialadenitis, retroperitoneal fibrosis and inflammatory aneurisms, and the term IgG4-related disease has been coined2-4. This rare disease can involve virtually all organs but thoracic involvement is even rarer and pleural involvement of the disease has not been reported yet in our country. We experienced a patient who presented with respiratory symptoms and pleural mass on chest X-ray, and finally diagnosed as IgG4-related disease involving pleura after surgical resection. We report this case with review of the pertinent literature.
A 66-year-old male was referred from a local clinic with one-week history of cough and sputum, and abnormal chest X-ray findings. He was a current smoker (one pack per day for 40 years) and had been diagnosed as Alzheimer type dementia. Medications for the dementia included donepezil, sodium valproate and quetiapine. He had no history of diabetes, hypertension and tuberculosis. He denied body weight loss or night sweating. He had no pleuritic chest pain or dyspnea.
On admission, he was acutely ill-looking. Blood pressure was 120/80 mm Hg, body temperature was 37.5℃, pulse rate was 66/min, and respiratory rate was 20/min. The lymph node in the neck was not palpated. Physical examination of the chest revealed crackle on right lower lung field but heart sound was normal without murmur. Complete blood count results were hemoglobin 12.7 g/dL, white blood cell (WBC) count 11,750/µL (neutrophils 76%, lymphocytes 21%, monocytes 2%, and eosinophils 1%), and platelet count 309,000/µL. C-reactive protein (CRP) was 34.5 mg/L (reference, 0.00-5.0 mg/L). Liver function test, blood urea nitrogen and glucose were normal. Urine analysis showed no abnormalities. Gram staining with culture, acid fast bacilli (AFB) smear and polymerase chain reaction for AFB of sputum were all negative. The urine pneumococcus antigen was also negative.
Chest X-ray revealed a pneumonic infiltration at right lower lobe (RLL) and a pleura-based mass at right upper hemithorax (Figure 1). Chest computed tomography (CT) scan showed a consolidation in RLL and a 3.0×1.3 cm-sized ovoid shaped pleural mass with contrast enhancement surrounding normal lung parenchyma with clear margin (Figure 2).
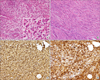
We started antibiotic therapy with a third-generation cephalosporin and macrolide considering the consolidation at RLL as pneumonia. After the treatment the respiratory symptoms and the consolidation on chest X-ray gradually improved. WBC count and CRP level also normalized. However, the pleural mass remained unchanged. We then performed video-assisted thoracoscopic surgery for the lesion. Grossly, the mass consisted of oval-shaped soft tissues 3.0×2.0×1.5 cm in size. Histopathologic examination revealed dense and diffuse lymphoplasmacytic infiltration, storiform fibrosis and some eosinophilic infiltration (Figure 3A, B). Immunohistochemical staining revealed numerous IgG and IgG4 positive cells (>50 per high power field) (Figure 3C, D). With the final diagnosis of IgG4-related disease, we then examined the serum IgG4 level, which was increased to 148.0 mg/dL (reference, 6.1-121.4 mg/dL). To identify possible synchronous lesions, we performed otolaryngologic examination and abdominal CT scan and no extrathoracic lesions were evident. The patient was discharged without further treatment at hospital day 14 and has been followed-up at an outpatient clinic without evidence of recurrence.
IgG4-related disease was initially recognized as a subtype of autoimmune pancreatitis1, but the histopathologic and serologic similarities with other diseases prompted this condition to a new disease category5. It has been described to involve virtually every organ system including the biliary tract, orbital cavity, salivary gland, retroperitoneum, aorta, skin, kidney and lung, with a histopathology that is strikingly similar regardless of the involved organs5.
Based on a recent consensus statement, the diagnosis of IgG4-related disease rests on the combined presence of the characteristic histopathology and increased IgG4-positive plasma cells6. The three major histopathologic features are dense lymphoplasmacytic infiltrate, fibrosis arranged at least focally in a storiform (irregularly whorled) pattern and obliterative phlebitis. In most instances, a confident diagnosis requires the presence of two of these major features. Although eosinophilic infiltration is often apparent, it is not diagnostically specific or sensitive. In the present case, major histopathologic features except obliterative phlebitis, and eosinophilic infiltration were noted. Quantitative assessment of IgG and IgG4 staining is essential for both differential diagnosis from other diseases and confirmation of IgG4-related disease. More than 50 IgG4-positive plasma cells per high power field strongly suggest the disease with high specificity6. Serum IgG4 level is elevated in only about 30% of the patients and the range varies widely, which lacks clinical value7,8.
Few epidemiological studies on the IgG4-related lung and pleural disease have been reported, but it is more common in males (81%) and in more elderly individuals (over 50 years old)9. The symptoms are nonspecific and the majority of patients are identified during routine medical check10. A study reported that about 43% of the patients with IgG4-related lung and pleural disease had extrathoracic lesions9. In this case, the patient was incidentally found to have a pleural mass after presenting with respiratory symptoms due to pneumonia, and no extrathoracic lesion was found despite of an abdominal CT scan and otolaryngologic examination.
Radiologically, IgG4-related pulmonary disease can be classified into four subtypes: a solid nodular type having a solitary nodule resembling primary lung cancer; a bronchovascular type showing thickening of bronchovascular bundles and interlobular septa; an alveolar interstitial type showing honeycombing and bronchiectasis and a round-shaped ground-glass opacity (GGO) type characterized by multiple round-shaped GGO9,11. In case of pleural disease, a pleural mass is formed at both the visceral and parietal pleural, and sometimes can involve subpleural lung parenchyma9.
IgG4-related disease often requires differential diagnosis from other diseases. Some inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease and Castleman's disease have an increased number of IgG4-positive plasma cells. However, none of these conditions shows the characteristic histopathological features of IgG4-related disease. The lymphomas sometimes mimic IgG4-related disease especially in diffuse large B cell lymphoma. However, the majority of lymphocytes in IgG4-related disease are T lymphocytes whereas lymphoma can have both B- or T-cell colonality8. In addition, malignancies other than lymphoma can be associated with the increase in the number of the IgG4-positive cells. Cancer tissue can be infiltrated by these cells to various degrees. IgG4-positive plasma cell infiltration in malignant tissue is usually patchy and not associated with other typical histological features of IgG4-related disease6.
The recent findings of the association of IgG4-related disease with malignancy and allergic disease are noteworthy. Several cases of pancreatic cancer in autoimmune pancreatitis and lymphoma in chronic sclerosing dacryoadenitis have been reported12,13. In addition, one premalignant lesion (adenomatous hyperplasia) and one lung adenocarcinoma were reported among 21 patients with IgG4-related lung disease9. Moreover, many patients with IgG4-related pulmonary disease tend to have allergic predisposition, such as bronchial asthma, allergic rhinitis, drug allergy and peripheral blood eosinophilia13,14. Whether the disease has a predilection to become cancerous and the mechanism of the development of allergic tendency remain unclear.
Involvement of the vital organ(s) is the most important factor to start treatment. Not all cases with clinical manifestation need treatment and many asymptomatic cases only require careful clinical observation3. Glucocorticoid has been used as the first-line treatment and immunosuppressants including azathioprine or methotrexate are required on occasion. Although data on the long-term prognosis after treatment are lacking, a retrospective study with a 3-month regimen of glucocorticoid showed good initial clinical response15.
In conclusion, we report a case of IgG4-related disease presented as a pleural mass. IgG4-related lung and pleural disease is a recently recognized disease entity that has a variety of clinical manifestations. Clinical suspicion and pathologic confirm is essential for diagnosis. Therefore, it is important to understand the clinicopathologic features of the disease and IgG4-related disease should be considered as one of the differential diagnoses when encountering a pleural mass.
Figures and Tables
Figure 1
Chest radiography showed a well-defined nodular opacity in right upper hemithorax (arrow) and consolidation at right lower lobe (asterisk).
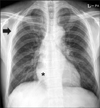
Figure 2
Chest computed tomography scan showed a consolidative lesion at right lower lobe (A) and a pleura-based mass with contrast enhancement surrounding normal lung parenchyma in right upper lobe (B).

Figure 3
Histopathologic examination showed dense infiltration of inflammatory cells consisting mainly of lymphocytes and plasma cells with some eosinophils (A), and characteristic storiform-type fibrosis (B) (A, B; H&E stain, ×200; inset in A, ×400). In immunohistochemical staining, numerous IgG- and IgG4-positive plasma cells were identified (C, D) (×200).
References
1. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001; 344:732–738.
2. Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004; 28:1193–1203.
3. Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Kuttner's tumor). Am J Surg Pathol. 2005; 29:783–791.
4. Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, Endo M, et al. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol. 2008; 32:197–204.
5. Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003; 38:982–984.
6. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192.
7. Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011; 23:108–113.
8. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012; 366:539–551.
9. Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2009; 33:1886–1893.
10. Kobayashi H, Shimokawaji T, Kanoh S, Motoyoshi K, Aida S. IgG4-positive pulmonary disease. J Thorac Imaging. 2007; 22:360–362.
11. Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009; 251:260–270.
12. Fukui T, Mitsuyama T, Takaoka M, Uchida K, Matsushita M, Okazaki K. Pancreatic cancer associated with autoimmune pancreatitis in remission. Intern Med. 2008; 47:151–155.
13. Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008; 32:1159–1167.
14. Kamisawa T, Anjiki H, Egawa N, Kubota N. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2009; 21:1136–1139.
15. Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009; 58:1504–1507.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download