Abstract
Malignant mesothelioma (MM) is the aggressive tumor of serosal surfaces. There are crude pathogenetic results regarding the biology of MM. Coordinated upregulations of p53 gene expression are shown in malignancies. We believed that there are changes in the p53 expression with transformation from reactive hyperplasia to MM. A 65-year-old male was admitted the hospital because of left pleuritic chest pains in 2004. Chest computed tomography (CT) results showed left pleural effusions with loculation and pleural thickening. Pathologic findings revealed reactive mesothelial hyperplasia. In 2008, the patient again felt left pleuritic chest pains. Chest CT showed progressive thickening of the left pleura. Pathologic diagnosis was atypical mesothelial hyperplasia. In 2011, chest CT showed progressive thickening of his left pleura. He was diagnosed with well-differentiated papillary mesothelioma. Serial change was analyzed by immunohistochemical staining for p53 of pleural tissues. There were no remarkable changes in p53 expressions during the transformation to MM.
Malignant mesothelioma (MM) is an aggressive tumor of serosal surfaces, including the pleura and the peritoneum1. Asbestos is the principal carcinogen associated with MM. Simian virus 40 (SV40), a DNA virus, has been implicated as a cofactor in the causation of MM1,2. There are crude pathogenetic results regarding the biology of MM. These include abnormal karyotypes (loss of chromosome 22, structural rearrangements of 1p, 3p, 9p, and 6q), growth advantage (platelet-derived growth factors A and B, epidermal growth factor and transforming growth factor β), immortalization by the action of telomerase, absence of tumor suppressor genes (Rb and p53, p16 and p14, and NF2-merlin), induction of antiapoptotic processes, increased angiogenesis (such as vascular endothelial growth factor), and matrix interactions1.
Histological examination remains the key for MM diagnosis3. Demonstration of stromal or fat invasion is the key feature in the diagnosis of MM4,5. However, the cytopathologist and surgical pathologist experience difficulties due to the many morphologic similarities between neoplastic cells and their benign counterparts3. Therefore, tumor markers have been used for MM diagnosis. The best known tumor markers of MM are epithelial membrane antigen (EMA), p53, and desmin. Other markers include GLUT-1, Ki-67 labeling, bcl-2, and p-glycoprotein4. High expression of desmin, low expression of EMA, and very low expression of p53 indicate benign mesothelial proliferation6.
Several immunohistochemical studies have been performed to differentiate between benign mesothelial proliferations and MM. p53 overexpression is a frequent feature of mesothelioma and is useful for differentiating between malignant and non-neoplastic mesothelial alterations4. However, there has been no report regarding changes in p53 expression in the same malignant pleural mesothelioma patient. We believe there is a change in p53 expression with transformation from reactive hyperplasia and atypical mesothelial hyperplasia to MM. Therefore, we analyzed the serial change in p53 expression by immunohistochemical staining in a patient with MM.
In 2004, a 65-year-old male presented with left pleuric chest pain. He had worked as a factory supervisor of asbestos-containing products for the past 20 years. His medical history was hypertension and diabetes mellitus. He was a former smoker, smoking 42 packs of cigarettes per year. Chest X-ray and computed tomography (CT) showed a moderate amount of left pleural effusion with some loculation, combined with mild pleural thickening and subtle enhancing area (Figure 1A, D, G), indicating a presumptive diagnosis of tuberculous pleurisy. However, cell differential count of pleural fluid showed a red blood cell count of 1,140/mm3, white blood cell count of 8,010/mm3, with 15% neutrophils, 4% lymphocytes, 65% macrophages, and 16% others. The patient's pleural adenosine deaminase level was 23 U/L. Aspiration cytology of pleural fluid was negative for malignant cells. Many clusters of reactive mesothelial cells and chronic inflammatory cells were seen in pleural fluid. Video-assisted thoracic surgery (VATS) was performed for a correct diagnosis, which showed thickened pleura. Pleura were partly resected. A grossly grayish white soft tissue section was taken. At that time, clinical, radiological and biochemical investigation did not identify malignancy in any organ, nor venous thromboembolism.
In 2008, the patient felt left pleuric chest pain again. Chest CT showed progressive thickening of the left pleura with nodularity (Figure 1B, E, H). According to VATS, the pleura appeared thickened. Therefore, left pleurectomy and wedge resection were performed.
In 2011, repeat CT showed progressive thickening of the left pleura (Figure 1C, F, I). A presumptive diagnosis was malignant transformation. The patient underwent exploratory VATS. A left pleurectomy and wedge resection in the left upper lobe were performed. The final pathologic diagnosis was well-differentiated papillary mesothelioma. The patient underwent Alimta/cisplatin combination chemotherapy for nine cycles and radiotherapy to left pleura.
In 2004, there was no definite evidence of MM, such as infiltration of deep tissue, obvious cytologic atypia, prominent cell grouping, or necrosis. The pathologic diagnosis was reactive mesothelial hyperplasia (Figure 2A, D). In 2008, there was diffuse fibrous thickening with fibrous exudate of the pleura. There was multifocal marked proliferation of mesothelial cells, sometimes forming papillary structures with myxoid fibrovascular cores. There was no evidence of invasion in the pleura or lung parenchyma. These findings were more suggestive of atypical hyperplasia of mesothelial cells than MM (Figure 2B, E). Immumohistochemical analysis of the patient's pleural tissue showed positive cytokeratin (CK), negative thyroid transcription factor 1, negative carcinoembryonic antigen, positive D2-40 (podoplanin), positive WT-1, and positive CK5/CK6 staining. These findings were more suggestive of mesothelial cells than epithelial cells of the gland.
In 2011, biopsy sections showed diffuse fibrous thickening with fibrous exudate of the pleura (Figure 2C, F). There was marked mesothelial hyperplasia with focal papillary structures. Cytologic atypia, prominent cell grouping and necrosis were not found. However, there was a superficial microinvasion with malignant potential. An asbestos fiber count on pleural tissue was not performed. Final pathologic diagnosis was well-differentiated papillary mesothelioma. At that time, immumohistochemical staining showed p53(+, focal), EMA(+), CK5/CK6(+), D2-40(+), HBME-1(+), and WT-1(-).
Immunohistochemical stain was performed to examine p53 expression in pleural tissue from 2004 and 2008. We got the informed consent to the patient about the use for research purposes. The corresponding paraffin-embedded cell blocks were cut 4-µm thick and stained with an anti-p53 antibody (PAb 240; ab26; 1:200, Abcam, Cambridge, UK). Incubation and pretreatment times were 2 hours. Appropriate positive and negative controls were included. Results revealed p53 focal positive staining with a diagnosis of reactive mesothelial hyperplasia in 2004 (Figure 3A, D). Additionally, p53 staining was focal positive with atypical mesothelial hyperplasia in 2008 (Figure 3B, E). In 2011, immunohistochemical stain revealed focal p53 staining with well-differentiated papillary mesothelioma (Figure 3C, F).
This case report focused on whether tumor suppressor gene expression changes during transformation from atypical hyperplasia to MM. This is the long-term follow-up study for serial measurements of pleural tissues in the same patient. However, in our analysis, we observed focal p53 immunohistochemical staining with reactive hyperplasia and atypical hyperplasia. This may mean that p53 overexpression exists not only in MM but also in reactive hyperplasia or atypical hyperplasia. There was no overt change in p53 immunohistochemical staining at diagnosis with MM. Although there was no remarkable change during transformation, this study was the first trial for serial observation about p53 expression in the same patient.
Generally, p53 staining is negative in atypical mesothelial hyperplasia and mesothelioma. p53 is also overexpressed more frequently in MM than reactive mesothelial proliferations, with a sensitivity ranging between 41% and 61%, and a specificity of 91%6,7,8,9. In the negative control, we observed focal positivity in 5% of reactive mesothelial cells. Due to the low sensitivity and specificity of p53, there were limitations in interpreting the results via immunohistochemical stains because wild-type p53 may be stained by the commonly used antibodies10,11.
p53 is a 53-kDa protein product of a tumor suppressor gene that regulates cell growth and inhibits cells from entering S-phase. Mutations in p53 are common in malignancies, and mutations in p53 lead to a prolonged half-life and accumulation of high amounts of the protein12,13. p53 overexpression was detected in 57.5% of patients in MM although no mutation was found in any of the studied exons. Moreover, the p53 gene mutations are rarely observed, although p53 overexpression is frequent14. This suggests the presence of other mechanisms for p53 inactivation in MM, such as sequestration of the wild-type p53 protein by other cellular proteins. The present results provide evidence for this mechanism because some of the present patients had marked cytoplasmic immunoreactivity for p53. The carcinogenic effect of SV40 is mediated by its tag antigen, which leads to inactivation of tumor suppressor gene production, such as that of p53 and Rb15. Tumor suppressor genes operate in various ways to block tumor growth. Although the two principal tumor suppressor genes, Rb and p53, are not commonly absent in MM, other molecules that are important in the Rb and p53 pathways are involved, particularly p16 and p14. Another gene product in the tumor suppressor gene pathway, NF2-merlin, is also important1.
Due to limitation of the quantitative immunohistochemistry method, there were limitations for this study. In previous studies, immunopositivity has been assessed in a semiquantitative manner (0, no staining; 1+, <25% cells positive; 2+, 26-75% cells positive; 3+, >75% cells positive) and staining intensity is low, moderate or high6. However, 2009 guidelines for pathologic diagnosis of MM suggest that weak or focal staining of less than 10% of the cells should be considered as being negative when interpreting a panel of stains4. Following this guideline, our results from three serial pleural tissues revealed ambiguous conclusions. The patient was diagnosed with well-differentiated papillary mesothelioma in 2011. We did not perform immunohistochemical analysis for p53 from pleural tissue in 2004 and 2008. Some people may believe that it would have been better to perform immunohistochemical staining, including p53 and EMA, at early presentation in 2004 and 2008. However, we diagnosed the patient with MM not because of p53 positive staining, but due to the presence of superficial microinvasion.
In summary, p53 was found to be overexpressed in MM. If MM is suspected clinically with atypical microscopic findings, p53 staining may helpful for diagnosis. However, p53 overexpression may exist not only in MM but also in reactive hyperplasia or atypical hyperplasia. Further studies are needed to determine changes in the amount of p53 during transformation for a larger population.
Figures and Tables
Figure 1
(A, D, G) In 2004, chest X-ray and computed tomography (CT) showed a moderate amount of left pleural effusion (D, white arrow) with loculation combined with mild pleural thickening and subtle enhancing area. (B, E, H) In 2008, chest CT showed progressive thickening of the left pleura with nodularity (E, white arrow). (C, F, I) In 2011, chest CT showed progressive pleural thickening with enlarged subpleural nodules (F, white arrowhead) in the left hemithorax.
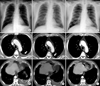
Figure 2
Pleural tissues diagnosed of reactive hyperplasia in 2004 (A, D). There was hyperplasia of mesothelial cells (arrowhead). In 2008, it was diagnosed for atypical mesothelial hyperplasia (B, E), there there were multifocal marked proliferations of mesothelial cells, sometimes forming papillary structures (arrowhead) with myxoid fibrovascular cores (arrow). In 2011, pleural tissues diagnosed of well-differentiated papillary mesothelioma (C, F). There was marked mesothelial hyperplasia with focal papillary structures (arrowhead) (A-C, H&E stain, ×100; D-F, H&E stain, ×400).
Figure 3
Serial pleural tissues with p53 immunohistochemical stain in a patient in 2004, p53 focal positive staining (arrowhead) with a diagnosis of reactive mesothelial hyperplasia (A, D); focal positive stain (arrowhead) with a diagnosis of atypical mesothelial hyperplasia in 2008 (B, E); focal p53 staining (arrowhead) with well-differentiated papillary mesothelioma in 2011 (C, F) (A-C, p53 immunohistochemical stain, ×200; D-F, p53 immunohistochemical stain, ×400).

References
1. Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005; 353:1591–1603.
2. Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, et al. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994; 9:1781–1790.
3. Hasteh F, Lin GY, Weidner N, Michael CW. The use of immunohistochemistry to distinguish reactive mesothelial cells from malignant mesothelioma in cytologic effusions. Cancer Cytopathol. 2010; 118:90–96.
4. Husain AN, Colby TV, Ordonez NG, Krausz T, Borczuk A, Cagle PT, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009; 133:1317–1331.
5. British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax. 2007; 62:Suppl 2. ii1–ii19.
6. Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium: a novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology. 2003; 43:231–238.
7. King J, Thatcher N, Pickering C, Hasleton P. Sensitivity and specificity of immunohistochemical antibodies used to distinguish between benign and malignant pleural disease: a systematic review of published reports. Histopathology. 2006; 49:561–568.
8. O'Kane SL, Pound RJ, Campbell A, Chaudhuri N, Lind MJ, Cawkwell L. Expression of bcl-2 family members in malignant pleural mesothelioma. Acta Oncol. 2006; 45:449–453.
9. Cagle PT, Brown RW, Lebovitz RM. p53 immunostaining in the differentiation of reactive processes from malignancy in pleural biopsy specimens. Hum Pathol. 1994; 25:443–448.
10. Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology. 1997; 30:403–418.
11. Scurry J, Duggan MA. Malignant mesothelioma eight years after a diagnosis of atypical mesothelial hyperplasia. J Clin Pathol. 1999; 52:535–537.
12. Angelopoulou K, Diamandis EP, Sutherland DJ, Kellen JA, Bunting PS. Prevalence of serum antibodies against the p53 tumor suppressor gene protein in various cancers. Int J Cancer. 1994; 58:480–487.
13. Lubin R, Schlichtholz B, Teillaud JL, Garay E, Bussel A, Wild CP. p53 antibodies in patients with various types of cancer: assay, identification, and characterization. Clin Cancer Res. 1995; 1:1463–1469.
14. Esposito V, Baldi A, De Luca A, Claudio PP, Signoriello G, Bolognese A, et al. p53 immunostaining in differential diagnosis of pleural mesothelial proliferations. Anticancer Res. 1997; 17:733–736.
15. Zekri AR, Bahnassy AA, Mohamed WS, Hassan N, Abdel-Rahman AR, El-Kassem FA, et al. Evaluation of simian virus-40 as a biological prognostic factor in Egyptian patients with malignant pleural mesothelioma. Pathol Int. 2007; 57:493–501.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download