Abstract
Patients with immune-mediated inflammatory diseases (IMIDs) are increasingly being treated with anti-tumor necrosis factor (TNF) agents and are at increased risk of developing tuberculosis (TB). Therefore, diagnosis and treatment of latent TB infection (LTBI) is recommended in these patients due to the initiation of anti-TNF therapy. Traditionally, LTBI has been diagnosed on the basis of clinical factors and a tuberculin skin test. Recently, interferon-gamma releasing assays (IGRAs) that can detect TB infection have become available. Considering the high-risk of developing TB in patients on anti-TNF therapy, the use of both a tuberculin skin test and an IGRA should be considered to detect and treat LTBI in patients with IMIDs. The traditional LTBI treatment regimen consisted of isoniazid monotherapy for 9 months. However, shorter regimens such as 4 months of rifampicin or 3 months of isoniazid/rifampicin are increasingly being used to improve treatment completion rates. In this review, the screening methods for diagnosing latent and active TB before anti-TNF therapy in patients with IMIDs will be briefly described, as well as the current LTBI treatment regimens, the recommendations for managing TB that develops during anti-TNF therapy, the necessity of regular monitoring to detect new TB infection, and the re-initiation of anti-TNF therapy in patients who develop TB.
South Korea has an intermediate tuberculosis (TB) burden: the annual incidence of TB is approximately 92 per 100,000 in the general population. Latent TB infection (LTBI) is expected to be present in about one third of general population.
Frequent immunosuppressive treatments and probably immune-mediated inflammatory disease (IMID) itself increase the risk of developing TB. In addition, the use of tumor necrosis factor (TNF) inhibitors increases more the incidence of TB in these patients. The risk of developing TB was higher in rheumatoid arthritis patients subjected to TNF inhibitor therapy than in otherwise healthy individuals and the incidence rate of TB increases as a result of TNF inhibitor therapy1,2. According to meta-analyses of studies on randomized controlled groups at home and abroad, the use of TNF inhibitors in inflammatory bowel disease (IBD) patients increased the relative risk of TB incidence by 2.52-fold3. For these reasons, most organizations in the world recommend screening for LTBI before starting therapy with anti-TNF agents4,5. Therefore, widespread screening and treatment of LTBI before initiation of anti-TNF treatment has dramatically reduced the incidence of TB6. In some areas, however, screening for TB before initiating treatment with TNF antagonists is rare7.
Patients treated with TNF inhibitors are at increased risk of developing TB, mostly through reactivation of LTBI8. However, diagnosis of LTBI is difficult, particularly in immunocompromised patients. Tuberculin skin tests (TSTs) are not sufficiently accurate9 and frequently yield false-positive results. Some of these limitations may be overcome through the use of new interferon-gamma (IFN-γ) releasing assays (IGRAs), including QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, VIC, Australia) and T-SPOT.TB (T-SPOT; Oxford Immunotec, Abingdon, UK), which detect cell-mediated IFN-γ responses to Mycobacterium tuberculosis-specific antigens. However, these IGRA tests are also affected by immunosuppression10.
In this review, we will address the diagnosis of latent and active TB before anti-TNF therapy, LTBI treatment regimens, what to do when TB develops during anti-TNF therapy, the necessity of regular monitoring for the detection of new TB infection, and the re-initiation of anti-TNF therapy in patients who develop TB.
To confirm active TB and LTBI prior to the initiation of TNF antagonists, clinical manifestations (history taking and physical examination), chest X-ray screening, and TB infection tests are essential (Figure 1). History taking includes the treatment history of previous treatments used for active TB or LTBI, bacillus Calmette-Guérin (BCG) vaccination history and current symptoms of suspected TB. A physical examination should be conducted, specifically targeting the parts of the body affected by active TB. Because active TB can be asymptomatic, chest X-ray screening is recommended for all patients. Tests for active TB need to be performed in patients suspected of having active TB based on clinical features and a chest-X-ray; treatment for LTBI is not recommended at this time. In cases with a history of adequate treatment for TB, TB infection tests are not considered to be clinically meaningful and treatment of LTBI is not performed unless patients have new infection (new contact with infectious TB patients)11. Despite a history of appropriate treatment for TB, the decision to treat LTBI is dependent on a new contact history with patients with infectious TB and their immune status. When the appropriateness of the treatment for previous TB is unclear, the decision to treat LTBI is dependent on the attending physician's decision. In cases with a history of inappropriate treatment for previous TB, the possibility of active TB should be excluded prior to the initiation of LTBI treatment. LTBI treatment is performed regardless of the results of TB infection tests, when fibrostreaky lesions, suggestive of spontaneously healed TB, are detected in the upper lobes on a chest X-ray. However, the presence of small calcified pulmonary nodules alone does not merit LTBI treatment, as these lesions rarely display viable organisms on autopsy12. Despite the presence of fibrotic lesions after pulmonary TB, the treatment of LTBI is not indicated for patients with a history of adequate treatment for previous TB.
The presence of LTBI is determined by performing TB infection tests in patients with no history of previous treatment for TB and a normal chest X-ray (no abnormal TB-related findings). Currently used tests can detect TB infection, including both active TB and LTBI, but they cannot differentiate between active TB and LTBI. Therefore, LTBI is diagnosed after excluding active TB in cases with a positive TB infection test. TB infection tests include TST and IGRA. There are 2 available IGRA commercial kits: QFT-GIT measures the concentration of released IFN-γ using an enzyme-linked immunosorbent assay technique; T-SPOT measures the number of cells producing and secreting IFN-γ. Both assays have a high specificity in diagnosing TB infection via their use of M. tuberculosis-specific antigens. These antigens are absent in the BCG strain. Conversely, purified protein derivatives used in TST include antigens present in BCG strains. For this reason, high cross-reactivity decreases TST specificity in diagnosing TB infection. According to the results of previous studies, diagnostic sensitivity for TB infection is similar in both TST and IGRA in immunocompetent hosts. Several studies on IGRAs in immunocompromised patients reported that the T-SPOT test showed a high sensitivity for diagnosing TB infection13. The use of immunosuppressants in patients with IBDs resulted in a high false negative TST rate due to anergy14,15. In contrast, IGRA is less influenced by immunosuppressive medications16. Because increased sensitivity may lower specificity, an appropriate cutoff value needs to be chosen by considering clinical circumstances. In a study of 212 patients (114 Crohn's disease, 44 ulcerative cloitis, 10 indeterminate colitis, and 44 controls) assessed by both TST and IGRA, agreement between the 2 test methods was poor. In contrast to the QFT-GIT test, the TST was negatively influenced by immunosuppressive medications and BCG vaccination status15.
Due to the recent availability of IGRAs, the tests most appropriate for detecting TB infection have become somewhat controversial. Because the risk of developing TB increases in immunosuppressed patients, many experts agree that increasing sensitivity is more important than a slight decrease in specificity in detecting TB infection. Therefore, the use of TST alone is not appropriate for detecting TB infection in patients due to start anti-TNF therapy. Thus, IGRA alone or a combination of TST/IGRA is thought to be appropriate. The 2011 Korean Practice Guidelines for the Control and Management of TB recommended 3 tests (TST alone, IGRA alone, and the TST/IGRA dual screening method) equally in detecting TB infection in patients with immunosuppression17. However, TST alone is expected to be excluded in the revised version which is planned for publication in 2014 (Figure 2). The UK's National Institute for Health and Clinical Excellence guidelines also do not recommend "TST alone" for detecting TB infection in patients with immunosuppression18. When using the TST/IGRA dual screening method, either test positive strategy can be used for determining positive LTBI in patients with immunosuppression. Therefore, TST and IGRA can be performed at the same time, or either test can be performed first, and if the test result is negative, then the other test can be performed. However, if either test performed first shows a positive result, then the other test is considered unnecessary. Recent data derived from UK clinical research verified that the LTBI detection rate was markedly increased when 3 methods (clinical factors, TST, and IGRA) were used in combination19.
One of the disadvantages associated with IGRAs is a higher frequency of indeterminate results in patients with immunosuppression as compared to individuals without immunosupression. According to the results of previous studies, indeterminate QFT-G results accounted for 21.4% of all patients with immunosuppression and the rate of indeterminate results in patients with higher than 100/µL of CD4 positive cells was 2.8% as compared to 24% among human immunodeficiency virus (HIV)-positive patients with less than 100/µL of CD4 positive cells10,20. When QFT-GIT was repeated using serum remaining after stimulation with TB-specific antigens in the first test, the rate of indeterminate results was also high (93.8%)21 and when the QFT-G test was repeated 1 month later in the same patient, the indeterminate result rate was 83.3%22. Therefore, it would seem to be inappropriate to repeat an IGRA test when the first IGRA result is indeterminate. In this case, the presence of LTBI needs to be determined with TST results and clinical factors only.
Because false positive results of IGRAs are likely to occur when performing IGRAs three days after TST due to purified protein derivatives injected during TST, immediate IGRA is recommended within the TST result interpretation time (48-72 hours after TST) when TST is performed prior to IGRA23,24,25,26.
Nine months of daily isoniazid (INH) administration is the standard regimen for treating LTBI12. After completion of treatment, the occurrence of TB can be prevented in approximately 90% of cases. However, LTBI treatment cannot prevent the development of TB via new infection that can occur following LTBI treatment. For this reason, the decision to treat LTBI should be readdressed after new contact with patients with infectious TB. The major disadvantage of INH alone is the long duration of treatment, whereas the duration of standard treatment for active TB is shorter by 3 months. This difference is mainly attributable to the minimal effectiveness of INH in dormant bacilli. A shortened 6-month drug regimen for treating active TB is possible due to the effectiveness of rifampicin (RIF) and pyrazinamide (PZA) in treating latent bacilli. Therefore, the use of RIF and PZA are expected to replace INH and enhance treatment effectiveness over a shorten treatment period in patients with LTBI. In animal experiments, various regimens, including RIF, showed more outstanding bacterial disinfecting effects as compared to the use of INH alone27. Therefore, a 2-month regimen of the RIF/PZA combination was recommended as an LTBI treatment strategy in the year 2000 in the United States12. However, the combination of RIF and PZA was subsequently excluded as an approved LTBI treatment strategy after several reports of deaths from severe liver toxicity28. Currently, a 4-month regimen of RIF is recommend in addition to the use of INH alone in the United States12, while a 3-month regimen of the INH/RIF combination is recommended in the UK, based primarily on data derived from studies involving children18,29. By accommodating those regimens, a 9-month INH regimen, a 4-month RIF regimen, and a 3-month regimen of the INH/RIF combination are equally recommended in Korea17,30. Although a 3-month regimen of the INH/rifapentine (RPT) combination (once a week for a total of 12 intermittent treatment sessions) has been approved and recommended for treating LTBI in the United States since 2011, RPT is not yet available in Korea31.
When initiating LTBI treatment, the most important factor to consider is the exclusion of the possibility of active TB. If the possibility of active TB is not excluded, LTBI treatment should be suspended. Since both INH and RIF are medications associated with a risk of liver toxicity, underlying liver diseases should be assessed before initiating LTBI treatment. Regular liver function tests are unnecessary during LTBI treatment if there are no risk factors for hepatitis. Currently, there is no way to confirm that LTBI has been adequately cured following the completion of LTBI treatment. Although 1 study reported that negative conversion was seen in some patients by performing IGRA on a regular basis during anti-TB treatment or LTBI treatment32, repeated IGRA is not recommended during LTBI treatment because of high variability in repeated tests. After treating LTBI, even when patients come into contact with infectious TB patients, additional TB infection tests are not considered clinically meaningful and the decision whether to treat LTBI should be determined based on clinical factors alone.
The initiation of anti-TNF treatment is generally recommended after 3-4 weeks of LTBI treatment11,33. According to the UK guidelines, treatment with TNF antagonists is recommended after completing treatment for LTBI if spontaneously healed TB lesions are shown on a chest X-ray34. However, these recommendations are not evidence-based, but reflect only expert opinion. In an observational study, TB did not develop in any of the approximately 60 patients for whom anti-TNF therapy was initiated at less than 3-4 weeks of LTBI treatment (Shim et al. unpublished). There is always a risk of contact with infectious TB patients in nations with a high incidence of TB. Therefore, the diagnostic tests for TB such as TB infection tests or chest X-rays may be performed regularly in patients taking anti-TNF agents and having negative LTBI test results at screening35,36. However, the necessity of regular TB infection tests has not been universally recommended at present. Currently, an immediate visit to a hospital for examination is the most crucial step in cases of suspected TB symptoms or signs. To achieve this, a thorough education regarding TB-suggestive symptoms in patients taking anti-TNF agents is essential. One follow-up strategy that have been employed in our institution is to see patients 3 months and 9 months after the initiation of anti-TNF therapy and then at 1 year intervals.
It is possible that some cases of active TB could be detected as an incidental finding on a chest X-ray conducted during a regular check-up in asymptomatic patients, but this is limited to the early stage of TNF antagonist treatment. In many cases, TB-suggestive symptoms such as fever, rather than a planned regular follow-up, drove patients to hospitals after the initial stage of TNF antagonist therapy. Therefore, a short interval of regular follow-up visits is critical during the first several months of the initial stage of anti-TNF therapy in which TB is most likely to occur. Afterwards, patients need to visit the hospital if they experience symptoms or they may lengthen their check-up intervals.
The risk of developing TB is high in cases of close contact with infectious TB patients during TNF antagonist therapy. The likelihood of active TB needs to be examined with a chest X-ray. Because re-examination is not clinically meaningful if a positive result has already been obtained from TB infection test at screening, the decision to treat LTBI should be based on clinical factors, without performing TB infection test. In cases of a mild contact and non-close contact, physicians may elect to observe patients closely and not initiate LTBI treatment. If the TB infection test result was negative at screening before the use of a TNF antagonist, positive conversion should be confirmed by performing a TB infection test. However, LTBI treatment should be initiated immediately despite a negative result in close contacts, as positive conversion takes approximately 2-10 weeks (window period) after infection with TB bacteria. Therefore, TB infection tests should be repeated 8-10 weeks after the completion of contact with infectious TB patients. LTBI treatment should be continued if the repeated test result is positive and stopped if the result is negative.
The occurrence of TB cannot be completely prevented during the implementation of TNF antagonist therapy despite LTBI treatment. For this reason, the possibility of TB development always needs to be taken into account37. When diagnosed with active TB, anti-TB treatment should be initiated and TNF inhibitor treatment should be stopped. Rapid drug susceptibility tests should be performed if microbiological tests are positive to promptly identify drug resistance17. The treatment period for active TB is identical to that of ordinary TB patients. A paradoxical response consisting of a favorable reaction of TB bacteria to anti-TB drugs, but deteriorated chest X-ray findings or symptoms such as fever due to enhanced immune response, can occur with the recovery from immunosuppression after suspending TNF inhibitor therapy38. Therefore, identifying negative conversion is of great importance in bacteriologically-confirmed TB patients. The possibility of a paradoxical response should be taken into consideration when drug susceptibility is confirmed to be pan-susceptible but chest X-rays or symptoms show deterioration despite a favorable reaction of TB bacteria to anti-TB drugs. In severe cases, treatment with immunosuppressive medications such as corticosteroids should be initiated. In a case report, TNF inhibitor therapy successfully controlled paradoxical responses that occurred during anti-TB treatment39.
Treatment with TNF antagonist needs to be re-initiated at a certain point in patients that stopped TNF antagonist therapy due to the occurrence of active TB. In general, initiating TNF inhibitor therapy is safe after completion of successful anti-TB treatment11. If TNF inhibitor therapy is urgent, treatment with TNF antagonists can be re-initiated after the completion of intensive phase treatment (first 2 months of standard regimen) and confirmation of favorable treatment responses. Concurrent administration with steroids from the start of anti-TB treatment is recommend for central nervous system TB and TB pericarditis among active TB diseases40. The results of previous studies have shown that an acceleration of the rate of decrease of bacteria was observed when immunosuppressants such as steroids or TNF antagonists were combined with anti-TB treatment in HIV-positive patients with TB41,42. Thus, an immunosuppressive condition may be a minor problem if adequate TB treatment is implemented and TB is not severe. Therefore, the use of TNF antagonists may be considered from the beginning of anti-TB treatment in mild and drug-susceptible TB cases. Further studies are needed to clarify this issue.
In the past, the incidence of TB was so high that a positive acid-fast bacilli (AFB) smear was regarded as the confirmation of TB. Presently, however, a positive AFB smear is no longer considered indicative of TB due to the gradual increase in the incidence of nontuberculous mycobacterial (NTM) disease in recent years. The diagnosis of TB can be confirmed through mycobacterial culture and identification of the M. tuberculosis complex. In addition, M. tuberculosis and NTM can be differentiated within a few days by performing a nucleic acid amplification test (NAAT) on AFB smear-positive specimens. The US practice guidelines have recommended NAAT to distinguish NTM from M. tuberculosis in cases of positive AFB smear43. The Korean clinical practice guidelines for TB, to be published in 2014, are anticipated to recommend repeated (twice) sputum AFB smear/culture tests and one NAAT in patients with suspected pulmonary TB. Although it is difficult to discriminate between pulmonary TB and NTM pulmonary disease clinically, a considerably low prevalence of NTM pulmonary disease in young, healthy individuals with normal immune function is helpful in discrimination. The likelihood of NTM pulmonary disease is high with the detection of bronchiectasis and centrilobular nodules in the right middle lobe or in the lingular segment of the left upper lobe in the lung on a simple chest X-ray or a chest computed tomography. In contrast, TB-suggestive lesions in the upper lobes are hardly distinguishable from NTM pulmonary disease. Since patients with Crohn's disease receiving a TNF antagonist are mostly young, the incidence of NTM pulmonary disease in these patients is rare. Instead, the incidence of NTM pulmonary disease may be higher in middle-aged or elderly patients with rheumatoid arthritis among TNF antagonist users.
There have not been any domestic studies that have investigated the association of the incidence of NTM pulmonary disease in TNF antagonist users. According to an analysis of the MedWatch database released in 2004 by the US Food and Drug Administration, the incidence of TB was 5-10 times higher than that of NTM or other granulomatous infections among TNF antagonist users44. According to the 2008 Emerging Infection Network of the Infectious Diseases Society of America, the incidence of NTM disease was about twice as high as the incidence of TB45. Although clinical experience regarding NTM pulmonary disease is still insufficient in current TNF antagonist users, the guidelines of the American Thoracic Society recommend that TNF antagonists can be used based on an expert's opinion only if adequate NTM treatment is being performed46. However, it is difficult to judge if treatment is adequate, as the treatment success rate of NTM pulmonary disease is lower than that of TB, and that unlike TB, clinical reactions are unpredictable based on drug susceptibility test results (excluding clarithromycin). In an observational study, the clinical manifestations and the degree of progression of NTM pulmonary disease varied insignificantly from immunocompetent individuals despite the use of a TNF antagonist (Shim et al. accepted in European Respiratory Journal). Therefore, withholding the use of a TNF antagonist may be a safer approach until anti-NTM treatment is administered in some period of time, however, the use of a TNF antagonist combined with NTM treatment can be considered with close monitoring of clinical progress when TNF antagonist therapy is determined to be urgent.
The diagnosis and treatment of TB infection prior to the initiation of anti-TNF therapy are already standard for patients with IMIDs. Details are summarized in Table 117. Further research will be required in order to develop more accurate tests to detect TB infection and to find more effective LTBI treatment regimens. This review with similar contents was already published in the journal of Intestinal Research as a review article47.
Figures and Tables
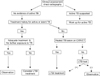 | Figure 1Algorithm for the selection of candidates requiring LTBI treatment. TB: tuberculosis; CXR: chest radiography; CT: computed tomography; LTBI: latent tuberculosis infection. |
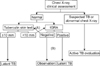 | Figure 2Diagnosis of LTBI in immunocompromised adults. The appropriate method, either a combination of TST/IGRA (A) or IGRA alone (B), should be chosen based on circumstances of individual patient. The negative LTBI test is not recommended with TST alone, and a positive TST is diagnosed as LTBI. IGRA: interferon-gamma releasing assay. TB: tuberculosis; IGRA: interferon-gamma releasing assay; LTBI: latent tuberculosis infection; TST: tuberculin skin test. |
Table 1
Summary of recommendations regarding LTBI diagnosis and treatment in patients on anti-TNF therapy17

References
1. Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007; 34:706–711.
2. Brassard P, Lowe AM, Bernatsky S, Kezouh A, Suissa S. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum. 2009; 61:300–304.
3. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013; 108:1268–1276.
4. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006; 130:940–987.
5. Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.
6. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005; 52:1766–1772.
7. Ke WM, Chen LS, Parng IM, Chen WW, On AW. Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha inhibitor treatment in Taiwan. Int J Tuberc Lung Dis. 2013; 17:1590–1595.
8. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–1104.
9. Ponce de Leon D, Acevedo-Vasquez E, Sanchez-Torres A, Cucho M, Alfaro J, Perich R, et al. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005; 64:1360–1361.
10. Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005; 172:631–635.
11. Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–1206.
12. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161(4 Pt 2):S221–S247.
13. Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002; 16:2285–2293.
14. Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004; 2:309–313.
15. Schoepfer AM, Flogerzi B, Fallegger S, Schaffer T, Mueller S, Nicod L, et al. Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol. 2008; 103:2799–2806.
16. Qumseya BJ, Ananthakrishnan AN, Skaros S, Bonner M, Issa M, Zadvornova Y, et al. QuantiFERON TB gold testing for tuberculosis screening in an inflammatory bowel disease cohort in the United States. Inflamm Bowel Dis. 2011; 17:77–83.
17. Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. Seoul: Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2011.
18. National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: National Institute for Health and Clinical Excellence;2011.
19. Singanayagam A, Manalan K, Sridhar S, Molyneaux PL, Connell DW, George PM, et al. Evaluation of screening methods for identification of patients with chronic rheumatological disease requiring tuberculosis chemoprophylaxis prior to commencement of TNF-alpha antagonist therapy. Thorax. 2013; 68:955–961.
20. Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir Res. 2006; 7:56.
21. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013; 187:206–211.
22. Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J. 2009; 33:812–815.
23. Igari H, Watanabe A, Sato T. Booster phenomenon of QuantiFERON-TB Gold after prior intradermal PPD injection. Int J Tuberc Lung Dis. 2007; 11:788–791.
24. Vilaplana C, Ruiz-Manzano J, Gil O, Cuchillo F, Montane E, Singh M, et al. The tuberculin skin test increases the responses measured by T cell interferon-gamma release assays. Scand J Immunol. 2008; 67:610–617.
25. Choi JC, Shin JW, Kim JY, Park IW, Choi BW, Lee MK. The effect of previous tuberculin skin test on the follow-up examination of whole-blood interferon-gamma assay in the screening for latent tuberculosis infection. Chest. 2008; 133:1415–1420.
26. van Zyl-Smit RN, Pai M, Peprah K, Meldau R, Kieck J, Juritz J, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009; 180:49–58.
27. Lecoeur HF, Truffot-Pernot C, Grosset JH. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am Rev Respir Dis. 1989; 140:1189–1193.
28. Centers for Disease Control and Prevention (CDC). American Thoracic Society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection United States, 2003. MMWR Morb Mortal Wkly Rep. 2003; 52:735–739.
29. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis. 2005; 40:670–676.
30. Yun JW, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Diagnosis and treatment of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. J Korean Med Sci. 2007; 22:779–783.
31. Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011; 365:2155–2166.
32. Higuchi K, Harada N, Mori T. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008; 13:468–472.
33. Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 27:19–30.
34. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005; 60:800–805.
35. Bermejo F, Algaba A, Chaparro M, Taxonera C, Garrido E, Garcia-Arata I, et al. How frequently do tuberculosis screening tests convert in inflammatory bowel disease patients on anti-tumour necrosis factor-alpha? A pilot study? Dig Liver Dis. 2013; 45:733–737.
36. Kim KH, Lee SW, Chung WT, Kim BG, Woo KS, Han JY, et al. Serial interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients treated with immunosuppressive agents. Korean J Lab Med. 2011; 31:271–278.
37. Bourikas LA, Kourbeti IS, Koutsopoulos AV, Koutroubakis IE. Disseminated tuberculosis in a Crohn's disease patient on anti-TNF alpha therapy despite chemoprophylaxis. Gut. 2008; 57:425.
38. Garcia Vidal C, Rodriguez Fernandez S, Martinez Lacasa J, Salavert M, Vidal R, Rodriguez Carballeira M, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis. 2005; 40:756–759.
39. Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008; 47:e83–e85.
40. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662.
41. Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004; 18:257–264.
42. Mayanja-Kizza H, Jones-Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis. 2005; 191:856–865.
43. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009; 58:7–10.
44. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004; 38:1261–1265.
45. Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM. Infectious Diseases Society of America Emerging Infections Network. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the Emerging Infections Network. Clin Infect Dis. 2008; 46:1738–1740.
46. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
47. Shim TS. Diagnosis and treatment of latent tuberculosis infection in patients with inflammatory bowel diseases due to initiation of anti-tumor necrosis factor therapy. Intest Res. 2014; 12:12–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download