Abstract
The early pulmonary consequences of inhalation injury are well documented; however, little is known about delayed pulmonary complications following thermal inhalation injury. Although thermal injury below the vocal cords is rare because of effective heat dissipation in the upper airway, inflammatory endobronchial polyps have previously been reported as a delayed complication associated with inhalation injury. We report an extraordinary case of tracheobronchial polyps in patients with smoke inhalation injury. This report shows the delayed development and natural course of tracheobronchial polyps following thermal injury.
Pulmonary injury from smoke inhalation is common in burn patients, significantly contributing to the morbidity and mortality of fire-related injuries1. The early pulmonary consequences of inhalation injury are well documented; however, little is known about delayed pulmonary complications following thermal inhalation injury. Although thermal injury below the vocal cords is rare because of effective heat dissipation in the upper airway2, inflammatory endobronchial polyps have previously been reported as a delayed complication associated with acute thermal injury3,4 or chronic smoke inhalation5. We report an additional case in which the lesions were studied histologically and regularly observed for six months.
A 21-year-old man was transferred to our hospital for evaluation of inhalation injury. He was exposed to superheated air and smoke while sleeping in his home. He was intubated for respiratory insufficiency and hypoxemia and treated with empiric antibiotics. He was extubated prior to transfer to our hospital for further evaluation. On admission, he was fully conscious and complained of throat irritation and hoarseness. Physical examination revealed a few superficial burns on the hand and knee. The oropharyngeal mucosa was erythematous without blisters. He had hoarseness and mild dyspnea but no stridor. Crackles were present on auscultation of both lower lung fields. Vocal cord inspection revealed edema only. His hemoglobin level was 11.7 g/dL (hematocrit, 34.9%), and his leukocyte count was 26,580/µg with segmented neutrophils being dominant. His arterial blood gas levels showed pH 7.45, PaO2 85.7 mm Hg, PaCO2 35.5 mm Hg, and saturation 98% while breathing inspired oxygen of 4 L/min via nasal cannula. Chest computed tomography showed multifocal, poorly defined, ground-glass opacities and diffuse bronchial wall thickening in both lungs. On the first hospital day, the patient was examined with a flexible bronchoscope to evaluate airway mucosa. His vocal cords were slightly edematous but moved normally. The tracheal and main bronchial mucosae were covered with thick whitish secretions. After saline irrigation, edematous and hyperemic mucosal changes were visualized (Figure 1A).
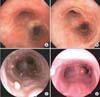
One month after the initial injury, the patient was asymptomatic except for persistent cough and stridor. Pulmonary function tests initially showed decreased vital capacity (44% of predicted); however, chest radiograph showed no abnormal findings. He was examined again with bronchoscopy. Numerous pale polypoid lesions were found in the vocal cords and throughout the tracheobronchial tree (Figure 1B). Corticosteroid (0.5 mg/kg/day) was administered for the treatment of his symptoms and polyps. After 7 days, the polyps had not changed on bronchoscopy, and steroid therapy was discontinued. The patient underwent biopsy of a polyp at the carina using a flexible bronchoscope, and histology revealed granulation tissue and fibrosis without specific findings compatible with viral infection (Figure 2). No treatment was given, and the progress of the polyps was followed by bronchoscopy. Three months after the initial injury, the lesions at the vocal cords regressed with fibrotic change, but the endobronchial polyposis had not changed. Background mucosa in the trachea appeared to be fibrotic in nature (Figure 1C). Six months after the initial injury, the lesions were slightly reduced with fibrotic changes in the trachea, but an additional exophytic polyp was seen at the right side of the carina (Figure 1D).
The pathophysiology of inhalation injury is complex, but it can be classified into three types: thermal injury to the upper airways, chemical injury to the tracheobronchial tree, and systemic poisoning due to carbon monoxide and/or cyanide1. However, inflammatory endobronchial polyps have previously been reported in the setting of acute thermal injury3,4. The etiology of endobronchial polyps has been explained by impaction of superheated particles at mucosal sites6. In addition, Rosenberg7 has postulated that inflammatory polyps form when a break in the bronchial mucosa occurs. This is followed by granulation tissue and subsequent replacement by fibrous tissue and epithelialization, as seen in our serial bronchoscopic findings.
There are few reports of endobronchial polyps following smoke inhalation, and the prevalence is unknown. This could be explained by the lack of bronchoscopic examination following thermal injury. Bronchoscopies are performed either during the initial insult or later when patients develop symptoms of airway stenosis2. We performed bronchoscopy when our patient developed chronic cough with stridor. Since these polyps appear to be a delayed complication following thermal injury, diagnosis of tracheobronchial polyps could not be made if bronchoscopy were only performed acutely after inhalation injury. Neither a uniform algorithm for assessing inhalation injury nor a reliable indicator of development of endobronchial polyps in patients with inhalation injury has yet been established.
In conclusion, we report an extraordinary case of tracheobronchial polyps after thermal injury in patients with smoke inhalation injury. This report shows the delayed development and natural course of tracheobronchial polyps following thermal injury.
Figures and Tables
Figure 1
A 21-year-old man with inhalation burn in the tracheobronchial wall. (A) Bronchoscopy showed edematous and hyperemic mucosal changes in the tracheobronchial wall at 2 weeks after the inhalation injury. (B) One month after the inhalation injury, numerous pale polypoid lesions were found in the tracheobronchial tree. (C) Three months after the initial injury, endobronchial polyposis had not changed, and background mucosa in the trachea appeared to be fibrotic in nature. (D) Six months after the initial injury, the lesions were slightly reduced with fibrotic change at the trachea, but an additional exophytic polyp was seen at the right side of the carina.
References
1. Heimbach DM, Waeckerle JF. Inhalation injuries. Ann Emerg Med. 1988; 17:1316–1320.
2. Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010; 12:53–61.
3. Adams C, Moisan T, Chandrasekhar AJ, Warpeha R. Endobronchial polyposis secondary to thermal inhalational injury. Chest. 1979; 75:643–645.
4. Williams DO, Vanecko RM, Glassroth J. Endobronchial polyposis following smoke inhalation. Chest. 1983; 84:774–776.
5. Smith RE. Endobronchial polyp and chronic smoke injury. Postgrad Med J. 1989; 65:785–787.
6. Freant LJ, Sawyers JL. Benign bronchial polyps and papillomas. Ann Thorac Surg. 1971; 11:460–467.
7. Rosenberg JC. Bronchial polyps of inflammatory origin. J Thorac Cardiovasc Surg. 1969; 57:848–852.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download