Abstract
Catamenial hemoptysis is a rare condition, characterized by recurrent hemoptysis associated with the presence of intrapulmonary or endobronchial endometrial tissue. Therapeutic strategies proposed for intrapulmonary endometriosis with catamenial hemoptysis consist of medical treatments and surgery. Bronchial artery embolization is a well-established modality in the management of massive or recurrent hemoptysis, but has seldom been used for the treatment of catamenial hemoptysis. We report a case of catamenial hemoptysis associated with pulmonary parenchymal endometriosis, which was successfully treated by a bronchial artery embolization.
Thoracic endometriosis is a rare disorder characterized by a presence of functional endometrial tissue within the pleura, the lung parenchyma or the airway1. The tissue is responsive to circulating sex hormones and clinical manifestations are related to the menstrual cycle. Clinically, thoracic endometriosis includes four well-recognized entities, namely, catamenial pneumothorax, catamenial hemothorax, catamenial hemoptysis, and lung nodules2. In catamenial hemoptysis, the source of bleeding is an endometrial implant located in the pulmonary parenchyma or in the airway3.
Various treatment modalities such as hormonal therapy, surgery or medical conservative treatment have been attempted, but controversies exist about optimal management of catamenial hemoptysis. Bronchial artery embolization (BAE) is a well established minimally invasive treatment modality for hemoptysis and few have been reported for the management of catamenial hemoptysis. Here, we describe a case of catamenial hemoptysis caused by pulmonary parenchymal endometriosis successfully treated with BAE.
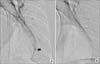
A 34-year-old married woman was admitted to pulmonary department with a 4-day history of hemoptysis. Hemoptysis occurred from the 3rd day of menstruation till 6th day and the total amount of hemoptysis was about 150 mL. She had no history of previous hemoptysis event. She had medical history of an appendectomy 20 years ago and pelvic inflammatory disease 2 years ago. She gave birth by normal spontaneous vaginal delivery (gravida 1, para 1) 10 years ago and she had not had a past history of obstetric or gynecological procedures before developing hemoptysis. Her medical history was otherwise unremarkable and she did not have a significant family history. She denied using smoking, excessive alcohol and illicit drugs. She had no complaints of weight loss, fever, dyspnea, palpitations, gastrointestinal symptoms or a history of bleeding. Her physical examination was within normal limits. Chest X-ray (Figure 1) had no abnormal findings. Chest computed tomography (CT) scan was performed on the 2nd hospital day, which was fourth day of menstruation and the CT scan showed a focal consolidation with adjacent ground glass opacity (GGO) in the posterior basal segment of the left lower lobe (Figure 2A), but there's no evidence of enlarged bronchial artery or vascular abnormality. Fiberoptic bronchoscopy showed a small amount of blood clot in the bronchi of left lower lobe. No endobronchial lesion was detected during the investigation. Bronchial washing fluid showed no acid fast bacilli, bacteria or abnormal cells. Hemoptysis was spontaneously resolved with the cessation of menstruation and did not recur during the rest of admission period. We assumed the illness as catamenial hemoptysis due to pulmonary endometriosis, and she was discharged. After discharge from the first admission, follow-up chest CT scan was performed for following up the lung lesion at 26th day of menstrual cycle. Previously noted focal consolidation with adjacent GGO lesion was almost disappeared and only a noncalcified 5-mm-sized nodule was left (Figure 2B). Four days passed and at the first day of the next menstrual cycle, hemoptysis recurred. The amount of hemoptysis ranged 100 to 150 mL and she was admitted again. Chest CT scan was performed and reappeared larger nodule with focal GGO was seen at the same location (Figure 2C). To control the bleeding, we decided to perform bronchial arteriography embolization. Digital subtraction technique with a digital subtraction angiography unit was used. Femoral artery was punctured, then thoracic aortography and followed selective left bronchial arteriography were done with a 5-Fr catheter. Diagnostic angiography showed a small nodular staining on left lower lung zone (Figure 3A) that corresponds with the lesion of previous chest CT scan. After determination of the pathologic vascularity, embolization was performed with 355 to 500 mm Contour polyvinyl alcohol particles. After embolization, control angiography showed occlusion of the artery feeding the lesion (Figure 3B). There was no more hemoptysis after the embolization. After discharge, she has been followed up for 5 months without hemoptysis. We diagnosed this case as catamenial hemoptysis because the hemoptysis events and size change of the lesion on chest CT scans were synchronized with the menstruation cycle, and both the CT scan and the bronchoscopy excluded other possible causes of hemoptysis.
Catamenial hemoptysis is a cyclic pulmonary hemorrhage that is synchronized with female menstruation, which is associated with the presence of intrapulmonary or endobronchial ectopic endometrial tissue. The mechanism regarding thoracic endometriosis is not fully understood and three theories have been proposed to explain the presence of intrathoracic endometrial implants: coelomic metaplasia, retrograde menstruation with subsequent transperitoneal-transdiaphragmatic migration of endometrial tissue and lymphatic or hematogenous embolization from the uterus or pelvis4. None of these theories can explain all the clinical manifestations, and the disease probably has a multifocal etiology. The theory of coelomic metaplasia is based on the concept that both endometrium and pleural mesothelium share the same embryologic origin. Pathologic stimuli induce precursor cells of the pleura into endometrial cells. The second theory is based on the concept that movement of fluids in the peritoneal cavity follows predictable patterns: namely "peritoneal fluid circulation." It implies a preferable flow direction from the pelvis to the right subdiaphragmatic area through the right paracolic gutter5. During the process of the flow, endometrial tissue could enter into the thorax through either congenital or acquired diaphragmatic defects5,6. However, these theories cannot explain the occurrence of intrapulmonary endometriosis. The last theory of transplantation of endometrium through lymphatic or vascular embolization can explain intrapulmonary endometriosis. Trauma or manipulation of uterine tissue would be a factor predisposing to microembolization. For example, the nationwide Korean report of 19 patients with catamenial hemoptysis showed that 16 (84%) patients had a history of obstetric or gynecological procedures before development of hemoptysis7. Also, in a study that followed 4 patients with catamenial hemoptysis, all patients had history of one or two dilatations and curettages before diagnosis of catamenial hemoptysis8. However, this patient had not had a past history of obstetric or gynecological procedures before developing hemoptysis.
The diagnosis of pulmonary parenchymal endometriosis is usually assumed on the basis of the clinical history and the exclusion of other causes of recurrent hemoptysis. Chest roentgenogram may reveal pulmonary opacities or nodular infiltrates, but findings could be normal even in a patient with current bleeding4. The diagnostic use of bronchoscopy is limited, because most cases of pulmonary endometriosis involve the distal pulmonary parenchyma rather than the mucosa of large bronchi and also the bleeding site may only be apparent during menstruation. Chest CT scan is useful for detection of the lesion and exclusion of other causes for hemoptysis. CT signs of pulmonary endometriosis include ill-defined or well-defined nodules, thin-walled cavities, bullous formations and ground glass opacities2. These lesions, which are expressions of the endometrial implants and/or secondary hemorrhage, may change in size during the menstrual cycle4,8. The lesion on CT scans in this patient also showed the characteristic change according to her menstrual cycle.
There is no guideline for the treatment of catamenial hemoptysis. Hormonal therapy has been considered as the first choice in patients with thoracic endometriosis. It includes oral contraceptives, progestational drugs, danazol, and gonadotropin-releasing hormone agonists which suppress the endometrial tissue. It has been proved to be effective in controlling symptoms, but controlled trials on the efficacy of these drugs are lacking. Also, heavy side effects of the hormonal therapy have been observed and symptoms often recur after discontinuation7. Moreover patients who consider pregnancy cannot take these drugs.
Medical conservative management could be another option, because most of the hemoptysis events associated with pulmonary emdometriosis are not lethal and most patients from case series are almost women of childbearing ages. In a study reported in Korea, 4 patients taken only conservative management had been followed approximately for five years and hemoptysis spontaneously disappeared after several episodes of minor bleeding8.
Surgical treatment has been advocated if medical treatment fails, intolerable drug-related side effects occur, or symptoms recur after the cessation of hormonal therapy3. Wedge resection or lobectomy can be applied to these cases according to the extent and location of the lesion. Video-assisted thoracoscopic surgery, endoscopic laser treatment9 or open surgery can be done7.
In our case we had observed the patient after the first episode, but the hemoptysis recurred in the next menstruation cycle. Since the amount of bleeding was more than that of the previous episode, we decided to try BAE prior to surgery. In general, BAE is a well-known alternative to surgery in the management of hemoptysis. Clinically, BAE has been widely applied for the treatment of hemoptysis caused by bronchiectasis, tuberculosis, aspergillosis, lung cancer or chest trauma. Possible rare complications of BAE are spinal cord injury, esophageal ulceration, stroke, bronchial infarction and transient chest pain. BAE may be more lifesaving and provide better long term control of recurrent bleeding and give better quality of life than medical conservative management alone does in massive or even if not massive but socially or physically recurrent troublesome hemoptysis10,11.
Blood supply of lung parenchymal endometriosis has not been well described. In some case reports or case series, pathologic findings of the removed pulmonary endometrial tissues revealed the presence of expanded bronchovascular bundles12 or thin-walled large capillaries or bronchial arteries13.
Despite these findings, BAE has not been frequently used to control catamenial hemoptysis. We found only one report from Kervancioglu et al.14, in which hemoptysis with multiple pulmonary endometriosis was successfully treated by BAE without recurrence for 3 months of follow-up. On the other hand, Katoh et al.15 found no abnormalities on bronchial and pulmonary angiograms in their clinically suspected pulmonary endometriosis patients. In this case, we found a small nodular staining in bronchial angiogram matched to the lesion on the chest CT scan. After BAE, the patient has not shown hemoptysis during the follow up period of 5 months with regular menses. In conclusion, we reported a patient of catamenial hemoptysis treated with BAE. This case suggests the possibility that BAE might be an alternative therapeutic strategy for the patient with catamenial hemoptysis of intrapulmonary endometriosis.
Figures and Tables
Figure 2
(A) Chest computed tomography (CT) scan performed on 4th day of menstruation shows focal consolidation with surrounding ground glass opacity in the posterior basal segment of the left lower lobe. (B) In the CT scan on the 26th day of menstruation, previously noted focal consolidation with surrounding ground glass opacity in the left lower lobe has been resolved. Residual small noncalcified nodule is noted. (C) In the CT scan on the 1st day of the next menstruation, focal ground glass opacity and nodule is noted at the same location as previous one.

References
1. Honore GM. Extrapelvic endometriosis. Clin Obstet Gynecol. 1999; 42:699–711.
2. Volkart JR. CT findings in pulmonary endometriosis. J Comput Assist Tomogr. 1995; 19:156–157.
3. Terada Y, Chen F, Shoji T, Itoh H, Wada H, Hitomi S. A case of endobronchial endometriosis treated by subsegmentectomy. Chest. 1999; 115:1475–1478.
4. Alifano M, Trisolini R, Cancellieri A, Regnard JF. Thoracic endometriosis: current knowledge. Ann Thorac Surg. 2006; 81:761–769.
5. Kirschner PA. Porous diaphragm syndromes. Chest Surg Clin N Am. 1998; 8:449–472.
6. Korom S, Canyurt H, Missbach A, Schneiter D, Kurrer MO, Haller U, et al. Catamenial pneumothorax revisited: clinical approach and systematic review of the literature. J Thorac Cardiovasc Surg. 2004; 128:502–508.
7. Kim CJ, Nam HS, Lee CY, Yum HK, Yang SH, Seo KH, et al. Catamenial hemoptysis: a nationwide analysis in Korea. Respiration. 2010; 79:296–301.
8. Ryu JS, Song ES, Lee KH, Cho JH, Kwak SM, Lee HL. Natural history and therapeutic implications of patients with catamenial hemoptysis. Respir Med. 2007; 101:1032–1036.
9. Puma F, Carloni A, Casucci G, Puligheddu C, Urbani M, Porcaro G. Successful endoscopic Nd-YAG laser treatment of endobronchial endometriosis. Chest. 2003; 124:1168–1170.
10. Mal H, Rullon I, Mellot F, Brugiere O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999; 115:996–1001.
11. Kim YG, Yoon HK, Ko GY, Lim CM, Kim WD, Koh Y. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Respirology. 2006; 11:776–781.
12. Flieder DB, Moran CA, Travis WD, Koss MN, Mark EJ. Pleuro-pulmonary endometriosis and pulmonary ectopic deciduosis: a clinicopathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls. Hum Pathol. 1998; 29:1495–1503.
13. Jelihovsky T, Grant AF. Endometriosis of the lung. Thorax. 1968; 23:434–437.
14. Kervancioglu S, Andic C, Bayram N, Telli C, Sarica A, Sirikci A. Bronchial artery embolization in the management of pulmonary parenchymal endometriosis with hemoptysis. Cardiovasc Intervent Radiol. 2008; 31:824–827.
15. Katoh O, Yamada H, Aoki Y, Matsumoto S, Kudo S. Utility of angiograms in patients with catamenial hemoptysis. Chest. 1990; 98:1296–1297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download