Abstract
Acute eosinophilic pneumonia (AEP) is a disease characterized by an acute febrile onset, eosinophilia in bronchoalveolar lavage fluid, and a dramatic response to corticosteroids. Although many studies have reported a close relationship between direct cigarette smoking and AEP, few studies have identified an association between passive smoking and AEP. Here, we report a case of AEP in a 19-year-old female with cough, fever, and dyspnea after 4 weeks of intense exposure to secondhand smoke for 6 to 8 hours a day in an enclosed area.
Acute eosinophilic pneumonia (AEP) is a disease characterized by acute onset of febrile respiratory symptoms, bilateral diffuse infiltrates on chest radiography (defined as an arterial oxygen pressure below 60 mm Hg or arterial oxygen saturation below 90% in room air), bronchoalveolar lavage (BAL) showing over 25% of eosinophils or eosinophilic pneumonia on lung biopsy, and absence of known causes of pulmonary eosinophilia1. Although the pathogenesis of AEP remains unclear, many studies have proposed a close relationship between direct cigarette smoking and the development of AEP1,2,3. We treated a 19-year-old female who had never smoked but had recently been exposed to massive amounts of cigarette smoke in an enclosed cafeteria, and developed clinical and pathologic features of AEP.
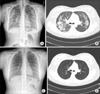
A 19-year-old female visited our hospital complaining of cough, sputum, fever and dyspnea for 5 days. She had been healthy and had no medical or medication history. She had never smoked, but she had spent 6 to 8 hours a day for the last 4 weeks in a cafeteria for a group study being exposed to environmental cigarette smoke. She was mildly dyspneic with an oxygen saturation of 85%, and other vital signs included a blood pressure of 122/85 mm Hg, a pulse rate of 100 beats per minute, a respiratory rate of 20 breaths per minute, and a body temperature of 37.5℃. On physical examination, inspiratory crackle was audible in both lung fields. Laboratory data revealed a peripheral white blood cell count of 10,130/mm3 (43.2% neutrophils, 36.9% lymphocytes, 10.1% monocytes, 9.1% eosinophils), hemoglobin of 13.0 g/dL, platelet count of 301,000/mm3, and a mild elevated serum C-reactive protein of 3.34 mg/dL. Other blood biochemical examination showed no specific abnormalities. Arterial blood gas analysis performed in ambient air showed pH 7.427, PaCO2 37.3 mm Hg, PaO2 49.8 mm Hg, HCO3 22.0 mEq/L, and oxygen saturation 83.6%. Initial chest radiographs showed diffuse bilateral interstitial infiltrates with ground glass opacity (Figure 1A). Computed tomography of the chest revealed symmetrical patchy consolidation and ground glass opacity with thickening of interlobular septa (Figure 1B). Rheumatoid factor, antinuclear antibody and anti-neutrophil cytoplasmic antibody were negative. The levels of immunoglobulin G (IgG), IgA, IgM, and complement 3 and 4 were in the normal range and the IgE level was 319 IU/mL. Her methacholine challenge test was negative.
BAL was performed on the first hospital day. The total cell count was 2.08×107 white blood cells per milliliter. The percentage of eosinophils had increased to 50% of white blood cells (Figure 2). BAL cultures were negative for bacteria, fungi, mycobacteria, Pneumocystis jiroveci, and viruses. Pulmonary function tests showed mainly a restricted pattern of respiratory distress with forced vital capacity (FVC) 1.82 L (53%), forced expiratory volume in one second (FEV1) 1.68 L (52%), FEV1/FVC 92%, and decreased diffusion capacity for carbon monoxide 10.1 mL/mm Hg/min (38%). Ultimately, our patient was diagnosed with AEP.
Therapy with daily intravenous methylprednisolone (1 mg/kg every 12 hours) was initiated following BAL, and the patient's condition including oxygen saturation and chest radiography dramatically improved in 3 days. The patient was discharged with oral methylprednisolone 32 mg (0.5 mg/kg/day for 1 month with subsequent tapering) after normalization of chest radiography. At 2 months after discharge, she had completely recovered from her respiratory complaints with normal lung function, and her chest radiographs showed complete resolution of the bilateral consolidations and ground-glass opacities (Figure 1C, D). The patient continued oral steroid therapy for about 3 months. Twelve weeks after the initial diagnosis, she had made a complete clinical recovery without relapse.
Our patient had been exposed to intense secondhand smoke in an enclosed cafeteria prior to the onset of AEP. Many studies have reported an association between direct smoking and development of AEP1,2,3,4. Some studies have used a cigarette-smoking challenge test to check for a direct association between smoking and development of AEP2,4. An epidemiologic study of AEP identified 18 patients among 183,000 US military personnel, and indicated that all of the patients were cigarette smokers, with 78% being new smokers with a mean duration of cigarette use of 1 month prior to disease onset3. Uchiyma et al.2 and Rhee et al.5 reported a relationship between smoking habits and idiopathic AEP in 33 patients and 137 patients, respectively. In those patients, the disease had not been induced by secondhand smoking.
Two studies have looked at an association between indirect cigarette smoking and AEP in the past6,7. In the first study, a 19-year-old male patient had been exposed to secondhand smoke from dormitory room and computer room for 8 days prior to onset of AEP7. His urinary cotinine on the 5th day of admission was 0 ng/mL, which indirectly showed that the patient had not been exposed to direct smoking. However, he had a brief history of smoking 1 year before the exposure to secondhand smoke which makes difference with this study. The second study studied a 22-year-old male who had been exposed to a large amount of cigarette smoke in an enclosed space for about 2 hours 2 days previously6. He had a past medical history of atopic dermatitis. Unlike previous two studies, the patient in this case had no history of direct smoking and allergic diseases, which might be associated with 4 weeks of relatively latent onset of AEP. Even more impressive, this is the first study to look at female with AEP associated with indirect cigarette smoking compared with previous two studies. Some reports have found that AEP is less likely to develop in females than males1,2,8, although there was no difference in the clinical course. Janz et al.8 examined the demographic of AEP, and reported that 65% of 83 patients with AEP were men and 35% were women.
The mechanism by which cigarette smoke induces AEP is yet to be determined. Cigarette smoke is a strong inflammatory stimulus that induces cytokines, such as interleukin (IL)-5, IL-6, and IL-7, as well as tumor necrosis factor (TNF), which may be the inciting event causing eosinophil-rich exudate in alveoli9,10. Thus, these cytokines are thought to be important factors that may induce AEP. Flouris et al.11 studied the effects of secondhand smoke on inflammatory markers. IL-4 and TNF-alpha were significantly increased in men and IL-5, IL-6, and interferon-gamma were increased in women after exposure to secondhand smoke. Therefore, passive smoking is thought to influence the inflammatory cytokines and chemokines that are important factors inducing AEP.
The patient's passive smoking was not objectively proven in this case. The patient in this case was a female who had never been smoked, and all the results of patient's self report, laboratory tests, cultures excluded the possible causative drugs, allergens, viral, bacterial, fungal and parasite infection, which could possibly cause AEP. Few days after admission, we realized that she had spent 6 to 8 hours a day for the last 4 weeks in a cafeteria for a group study, being exposed to cigarette smoking environment. We suspected that the only possible causative agent that could have induced AEP in this case to be the massive and intensive cigarette smoking environment. There are several ways to assess one's exposure to tobacco smoke such as taking measure of urinary nicotine and urinary cotinine, the metabolite of nicotine. However, in this case, checking the amount of urinary cotinine was meaningless since the half life of cotinine had already passed at that time. Although there was no direct evidence of patient's passive smoking, passive smoking might have induced AEP based on all circumstances.
This case report suggests that indirect smoking is as associated with AEP as direct smoking and that passive smoking may be injurious to young non-smoking subjects. The mechanism is not clear, but inflammatory cytokines and chemokines induced by indirect smoking may be important. Further comprehensive studies should be designed to evaluate the effect of passive smoking on AEP.
Figures and Tables
Figure 1
Chest radiography and computerized tomography (CT) scan on admission and 2 months after treatment. (A) Chest radiographs on admission revealing bilateral interstitial infiltrates and ground glass opacity. (B) Low dose chest CT on admission revealing symmetrical patchy consolidation and ground glass opacity with thickening of the interlobular septa. (C) Chest radiography at 2 months after treatment showing normalized lung. (D) Chest CT at 2 months after treatment also showing normalized lung.
References
1. Philit F, Etienne-Mastroianni B, Parrot A, Guerin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002; 166:1235–1239.
2. Uchiyama H, Suda T, Nakamura Y, Shirai M, Gemma H, Shirai T, et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008; 133:1174–1180.
3. Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004; 292:2997–3005.
4. Watanabe K, Fujimura M, Kasahara K, Yasui M, Myou S, Kita T, et al. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002; 41:1016–1020.
5. Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013; 41:402–409.
6. Komiya K, Teramoto S, Kawashima M, Kurosaki Y, Shoji S, Hebisawa A. A case of acute eosinophilic pneumonia following short-term passive smoking: an evidence of very high level of urinary cotinine. Allergol Int. 2010; 59:421–423.
7. Yoo KD, Jang W, Hong JH, Lee HP, Choi SJ, Choi SB. Acute eosinophilic pneumonia caused by passive smoking. Korean J Med. 2009; 77:508–511.
8. Janz DR, O'Neal HR Jr, Ely EW. Acute eosinophilic pneumonia: a case report and review of the literature. Crit Care Med. 2009; 37:1470–1474.
9. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996; 97:1366–1374.
10. Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996; 9:1989–1994.
11. Flouris AD, Metsios GS, Carrillo AE, Jamurtas AZ, Gourgoulianis K, Kiropoulos T, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med. 2009; 179:1029–1033.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download