Abstract
A nasal-type extranodal natural killer/T-cell lymphoma is considered an aggressive form of non-Hodgkin's lymphoma, with approximately half of all patients relapsing during the follow-up period, and most relapses occurring within the first 2 years of remission. Here we report an unusual case of a 42-year-old man who experienced recurrence in single pleura after 8 years of remission.
Extranodal natural killer (NK)/T-cell lymphoma is a subtype of lymphoma that is derived from NK cells and is relatively more common in Asia than in Western countries1. NK/T-cell lymphoma considered an aggressive form of non-Hodgkin's lymphoma (NHL)2. Approximately half of all patients relapse during the follow-up period, and relapsed NK/T-cell lymphoma patients often show a fulminant clinical course that is refractory to conventional chemotherapy. Most relapses of NK/T-cell lymphoma occur within the first 2 years of remission3. However, rare cases of relapse in NK/T-cell lymphoma, nasal type have been described after many years of remission4. Such late relapses are so rare that practically nothing is known about them.
We describe an unusual case of a 42-year-old man who experienced recurrence after 8 years of remission. The patient presented with pleural effusion involving Epstein-Barr virus (EBV)-positive NHL cells of the NK/T-cell lineage.
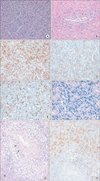
A 42-year-old man was admitted to the hospital after experiencing fever and night sweating for 2 weeks. He had visited another hospital 1 month earlier because of pain on the left side of his chest. At this hospital, he underwent chest radiography and computed tomography (CT), which showed a left pleural effusion. Diagnostic thoracentesis was performed, yielding 200 mL of clear, yellow-colored pleural fluid. Analysis of the pleural fluid revealed an exudative pattern; a protein level of 5.3 g/dL (serum protein level, 7.5 g/dL), a lactate dehydrogenase (LDH) level of 273 IU/L (serum LDH level, 342 IU/L), a glucose level of 61 mg/dL, an albumin level of 3.3 g/dL, and a pH of 7.3. The pleural fluid cell count was 2,560 cells/mL, with 10% polymorphonuclear cells, 90% lymphocytes, and an adenosine deaminase (ADA) concentration of 82 IU/L. No malignant cells were observed on cytological analysis. Bacterial, fungal, and mycobacterial cultures of the pleural fluid were also negative. The patient was diagnosed with tuberculous pleurisy and was administered anti-tuberculosis medications. After treatment, the pleural effusion and chest pain disappeared and the patient was discharged from the hospital. However, 2 weeks later, he developed new symptoms of fever, chills, and night sweating, which caused worry because of the patient's past illness. Eight years ago, he was diagnosed with extranodal NK/T-cell lymphoma, nasal type. He had been successfully treated with treatment with a combined of chemotherapy and radiotherapy resulting in a complete remission without recurrence. Upon admission, the patient's vital signs were stable, except for the presence of fever. In addition, laboratory findings were within the normal range. A chest radiograph showed findings consistent with a small amount of pleural effusion (Figure 1A). A chest CT scan revealed an heterogeneously enhanced-mass to the left of the eight thoracic spines (Figure 1B). A subsequent 18F-fluorodeoxyglucose (18F-FDG)-positron emission tomography revealed FDG activity at the same site (Figure 1C). A biopsy using video-assisted thoracic surgery was performed. Subsequent analysis by microscopy showed that the pleural tissue was diffusely infiltrated with atypical lymphoid cells (Figure 2A). It was CD3 +, CD56 +, granzyme B +, and EBV + (Figure 2C-F). These histological features were corresponded with the previous biopsy from nasal cavity eight years ago (Figure 2G, H). This led us to diagnose the patient's condition as recurrent pleural recurrence of extranodal NK/T-cell lymphoma. He has been been undergoing sequential chemotherapy followed by autologous hematopoietic stem cell transplantation.
According to the new World Health Organization classification, extranodal NK/T-cell lymphoma, nasal type is classified as a subtype of peripheral T-cell lymphoma5. NK/T-cell lymphomas show a specific geographical predilection for Asia. And in Korea, 9-12% of all NHLs are NK/T-cell lymphomas6. NK/T-cell lymphomas are considered an aggressive form of NHL, approximately 50% of patients relapse during the follow-up3. Common relapse sites include nasal sites and its adjacent structures; however, relapse also occurs at distant sites throughout the whole body.
In this case study, NK/T-cell lymphoma recurred with tuberculosis-like symptoms and left pleural effusion after 8 years of remission. The tumor clinically mimicked pulmonary tuberculosis as it presented with pleural effusion without lymphadenopathy, organomegaly, or an extranodal mass. Pleural fluid analysis revealed exudates with a predominance of lymphocytes and high ADA levels. On the basis of these results, the patient was diagnosed with tuberculosis and the initial symptoms disappeared after anti-tuberculosis treatment. However, these symptoms reappeared during the anti-tuberculosis treatment, and the final diagnosis was NK/T-cell lymphoma, nasal type, which recurred 8 years after the first remission.
Tuberculous pleurisy is an important differential diagnosis when assessing lymphocytic pleural effusions with high ADA levels in patients with pleural effusion. However, pleural effusion is a relatively common finding in patients with NHL, occurring in up to 20% of cases7. However, the rate of positive cytological findings varies widely (22-94%)8. Tuberculous pleurisy accounts for 25% of all cases of pleural effusion9. Although a definitive diagnosis of tuberculous pleurisy relies on polymerase chain reaction (PCR), a stain or culture of tubercle bacilli from pleural fluid, or pleural biopsy, these tests have limited sensitivity10. A diagnosis can also be established with reasonable certainty on the basis of elevated ADA levels in pleural fluid or pathologic findings in the pleura, including granulomas and Langerhans-type giant cells. However, patients with pyothorax, rheumatoid arthritis, malignant lymphoma, or other maliginancies may also exhibit elevated ADA levels11. Although pleural effusion was controlled and the patient was afebrile after anti-tuberculosis therapy, the primary etiology of elevated ADA levels in the presented case was presumed to be NK/T-cell lymphoma.
In Korea, the prevalence of pulmonary tuberculosis has been high until recently, and tuberculous pleurisy is also common. Wu et al.12 reported that the hazard ratio of tuberculosis was 3.22 in patients with hematological malignancies, including NHL and leukemia, compared to healthy individuals.
In rare cases, the co-existence of malignant lymphoma and tuberculosis has been reported13,14. Most of these cases were of pulmonary tuberculosis or lymph node tuberculosis. Reports of co-existing malignant lymphoma with tuberculous pleurisy are rare. However, considering the clinical findings, including negative findings for tuberculosis on PCR of pleural effusion and no effect of anti-tuberculosis treatment, it can explained that the origin of the pleural effusion was not tuberculosis but NK/T-cell lymphoma.
Although pleural fluid ADA analysis is very easy, cheap, and highly sensitive and specific test for diagnosis of tuberculous pleurisy, we should know that it can be increased in some of malignancy such as lymphoma, lung carcinoma, colorectal carcinoma, acute lymphoid leukemia, and mesotheolioma11. So we should pay attention to false positive increase of pleural ADA activity in tuberculosis pleurisy diagnosis. The case presented here demonstrated the importance of considering the possibility malignancy in exudative pleural effusion patient with a predominance of lymphocytes and high ADA levels.
Figures and Tables
Figure 1
(A) A chest radiograph showing costophrenic angle blunting, consistent with a small amount of pleural effusion. (B) A chest computed tomography (CT) scan showing an enhanced heterogeneous mass-like lesion in the left eight paravetebral area. (C) 18F-fluorodeoxyglucose (FDG)-positron-emission tomography revealed an intense FDG uptake at the same site as on the CT.
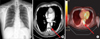
Figure 2
Pleural involvement of extranodal natural killer/T-cell lymphoma, nasal type. (A) Atypical lymphocytes of small to medium size are diffusely infiltrated in pleural tissue (H&E stain, ×200). (B) The infiltrate shows an angiocentric growth pattern (H&E stain, ×200). (C-E) The neoplastic cells shows positive reactivity for CD3 (C, ×200), CD56 (D, ×200), and granzyme B (E, ×200) by immunohistochemical staining. (F) Epstein-Barr virus in situ hybridization reveals nuclear positivity blue color (×200). (G) The initial biopsy from the nasal cavity showed proliferation of atypical lymphoid cells with extensive necrosis (asterisk), angiocentric growth pattern (arrows) and mild pleomorphism (H&E stain, ×200). (H) The neoplastic cells revealed immunoreactivity for CD56 antibody (×200).
References
1. Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24:612–618.
2. Kim GE, Cho JH, Yang WI, Chung EJ, Suh CO, Park KR, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000; 18:54–63.
3. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005; 19:2186–2194.
4. Au WY, Kim SJ, Yiu HH, Ngan RK, Loong F, Kim WS, et al. Clinicopathological features and outcome of late relapses of natural killer cell lymphomas 10-29 years after initial remission. Am J Hematol. 2010; 85:362–363.
5. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009; 523–531.
6. Kim J, Kim EY, Lee SK, Kim DI, Kim CH, Kim SH, et al. Extranodal nasal-type NK/T-cell lymphoma: computed tomography findings of head and neck involvement. Acta Radiol. 2010; 51:164–169.
7. Berkman N, Breuer R, Kramer MR, Polliack A. Pulmonary involvement in lymphoma. Leuk Lymphoma. 1996; 20:229–237.
8. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006; 34:335–347.
9. Valdes L, Alvarez D, Valle JM, Pose A, San Jose E. The etiology of pleural effusions in an area with high incidence of tuberculosis. Chest. 1996; 109:158–162.
10. Valdes L, Alvarez D, San Jose E, Penela P, Valle JM, Garcia-Pazos JM, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. 1998; 158:2017–2021.
11. Lee YC, Rogers JT, Rodriguez RM, Miller KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest. 2001; 120:356–361.
12. Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, et al. Aerodigestive tract, lung and haematological cancers are risk factors for tuberculosis: an 8-year population-based study. Int J Tuberc Lung Dis. 2011; 15:125–130.
13. Falagas ME, Kouranos VD, Athanassa Z, Kopterides P. Tuberculosis and malignancy. QJM. 2010; 103:461–487.
14. Lanjewar DN, Lanjewar SD, Chavan G. Coexistent lymphoma with tuberculosis and Kaposi's sarcoma with tuberculosis occurring in lymph node in patients with AIDS: a report of two cases. Indian J Pathol Microbiol. 2010; 53:551–554.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download