Abstract
Background
Apoptosis plays a role in the development of pleural effusion. Caspase-cleaved cytokeratin 18, a marker for epithelial cell apoptosis, was evaluated in pleural effusion.
Methods
A total of 79 patients with pleural effusion were enrolled. The underlying causes were lung cancer (n=24), parapneumonic effusion (n=15), tuberculous effusion (n=28), and transudates (n=12). The levels of M30, an epitope of caspase-cleaved cytokeratin 18, were measured in blood and pleural fluids using enzyme-linked immunosorbent assay along with routine cellular and biochemical parameters. The expression of M30 was evaluated in the pleural tissues using immunohistochemistry for M30.
Results
The M30 levels in pleural fluid were significantly higher in patients with tuberculosis (2,632.1±1,467.3 U/mL) than in patients with lung cancer (956.5±618.5 U/mL), parapneumonic effusion (689.9±413.6 U/mL), and transudates (273.6±144.5 U/mL; all p<0.01). The serum levels were not significantly different among the disease groups. Based on receiver operating characteristics analysis, the area under the curve of M30 for differentiating tuberculous pleural effusion from all other effusions was 0.93. In the immunohistochemical analysis of M30, all pathologic types of cancer cells showed moderate to high expression, and the epithelioid cells in granulomas showed high expression in tuberculous pleural tissues.
Apoptosis plays an important role in the pathogenesis of various diseases1-3. Therefore, an understanding of apoptosis is important to gain an understanding of various pathophysiological processes. It can also be utilized as a clinical marker for the diagnosis and assessment of diseases. The activity of apoptosis can be assessed by evaluating structural changes in cells, intermediary signaling molecules, and byproducts of apoptosis4. Many apoptosis-related parameters have shown utility as clinical markers for both benign and malignant diseases. In lung cancer, genetic and signaling molecules such as p53, the Bcl-2 family, and epidermal growth factor receptor are useful markers for diagnosis and treatment5,6. The Fas/Fas ligand system, which is closely associated with apoptosis, has been shown to be a valid marker for inflammatory pulmonary diseases such as tuberculosis7.
Cytokeratin is an intermediate filament protein constituting the cytoskeleton of epithelial cells. In apoptosis, cytokeratins are cleaved by caspases, and among them, cytokeratins 8, 18, and 19 have shown promise as clinical markers8-10. Because cytokeratins are exclusively expressed in epithelial cells, they have been utilized as markers for apoptosis in carcinomas such as breast cancer, lung cancer, colon cancer, and ovarian cancer11-13. Cytokeratin 18, a type I intermediate filament protein, is useful for evaluating apoptosis in epithelial cell-originated malignancies because it is a major component of epithelial cells but is not contained in other cell types such as mesenchymal or bone marrow-derived cells14. M30 is a neoepitope of caspase-cleaved cytokeratin 18 (CK18-Asp396-NE), and an enzyme-linked immunosorbent assay (ELISA) assay using a monoclonal antibody for M30 has been developed for evaluating epithelial apoptosis12,15-17. Measurement of apoptosis by this M30 ELISA assay is useful for the diagnosis and assessment of disease status in patients with lung cancer17,18. We previously reported the utility of M30 as a clinical marker for idiopathic interstitial pneumonia in benign lung diseases, and for the severity of lung injury in acute respiratory distress syndrome14,19. Thus far, M30 has mostly been evaluated in the blood and tissues of patients. We hypothesized that it would also be useful for evaluating pleural effusion because apoptosis plays a role in the pathogenesis of pleural effusion20,21.
We investigated the clinical significance and diagnostic utility of measuring M30 levels for evaluating epithelial cell apoptosis in pleural effusion of various causes.
A total of 79 patients were enrolled in this study from November 2010 to April 2011 at Ajou University Hospital. Pleural fluids were classified into exudates and transudates according to Light's criteria22. The causes of pleural effusion were diagnosed by the clinical manifestations, radiological findings, pathologic findings, and biochemical and cellular analyses of pleural fluids. Patients were diagnosed as having tuberculous effusion when they met any of the following criteria: Mycobacterium tuberculosis isolated from pleural fluid or pleural tissue; granulomatous inflammation with caseous necrosis in the pleural tissue with positive staining for acid-fast bacilli; granulomatous inflammation with caseous necrosis in the pleural tissue that did not stain for acid-fast bacilli, but showed a response to antituberculous treatment; or positive sputum culture growth of M. tuberculosis in the presence of pleural effusion. Malignant pleural effusion was diagnosed by identifying malignant cells using pleural fluid cytology or pleural tissue examination. Pleural effusion was considered parapneumonic if the causative organism was found by specimen culture or if it was accompanied by bacterial pneumonia, a lung abscess, or bronchiectasis in the presence of negative tuberculosis and malignancy evaluations and reinforced by a clinical response to treatment.
This study was approved by the Institutional Review Board of Ajou University School of Medicine. All subjects provided written informed consent for the collection of samples and subsequent analysis.
Samples of blood and pleural fluid from all patients were collected. Thoracentesis was performed in the usual manner, and the pleural tissue samples were obtained in 70 cases by blind biopsies with Abram's needle (n=58) or thoracoscopy (n=12). A portion of each pleural effusion sample was submitted for acid-fast staining, cytologic examination, and measurement of pH, protein, albumin, lactate dehydrogenase (LDH), and glucose. Total, white, and differential cell counts (Giemsa stain) were obtained by counting at least 200 cells under a light microscope. Another portion of the sample was centrifuged at 3,000 g per minute for 15 minutes, and the supernatants were frozen at -70℃.
The levels of M30 in sera and pleural fluids were measured using an M30-Apoptosense ELISA kit according to the manufacturer's instructions (Peviva AB, Bromma, Sweden).
For immunohistochemistry, five cases were selected for tuberculous pleural effusion and parapneumonic effusion, respectively, and four cases for each cell type of the malignant effusions. Immunohistochemical studies were conducted using the streptavidin-biotin-peroxidase method (UltraVision LP Large Volume Detection System; Thermo Fisher Scientific, Fremont, CA, USA). Briefly, sections (4 µm thick) were cut from paraffin-embedded material, deparaffinized with xylene, and rehydrated through a graded series of ethanol. After the inhibition of endogenous peroxidase, the sections were exposed to primary antibody at 4℃ overnight. Mouse monoclonal anti-human caspase-cleaved keratin 18 neoepitope M30 antibody (1:100, Peviva AB) was used as the primary antibody. The immunoreactive proteins were visualized with 3,3-diamino-benzidine, and the sections were counterstained with hematoxylin. For the negative controls, sections were incubated with nonimmunized mouse IgG instead of specific monoclonal antibodies and then processed according to the above procedure. Two pathologists evaluated the immunohistochemical staining patterns. First, the types of cells expressing M30 were determined. Thereafter, immunoreactivity for M30 was graded semiquantitatively as low, weak, moderate, or high.
All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). Numerical data are expressed as mean±SD. The Mann-Whitney U test and the Kruskal-Wallis nonparametric ANOVA test were used for comparisons among groups. Spearman's rank coefficient correlation was used to analyze correlations between parameters. A discriminant analysis was performed using receiver operating characteristic (ROC) analysis. The area under the curve (AUC) was obtained, and the optimal cut-off values were determined by maximizing the sum of the sensitivity and specificity. A value of p<0.05 was considered statistically significant.
The study population comprised 24 patients with malignant effusion due to lung cancer (adenocarcinoma, 15; squamous cell carcinoma, 5; small cell carcinoma, 4), 15 with parapneumonic effusion, 28 with tuberculous effusion, and 12 with transudates.
There were no differences in the total protein level or the total cell count in pleural fluid among the groups with exudates. The neutrophil fractions and LDH levels were higher in parapneumonic pleural effusions than in malignant or tuberculous effusions (Table 1).
The serum M30 levels were not significantly different among the disease groups (malignant effusion, 173±93 U/mL; parapneumonic pleural effusion, 160±106 U/mL; tuberculous effusion, 140±80 U/mL; transudates, 117.2±62.8 U/mL), although the transudates showed lower levels than those of all exudates combined (Figure 1).
The level of M30 in pleural fluids were significantly higher in exudates (1,597±1,356.9 U/mL) than in transudates (273.6±144.5 U/mL) (p<0.01). The level of M30 in pleural fluids were significantly higher in tuberculosis (2,632.1±1,467.3 U/mL) than in lung cancer (956.5±618.5 U/mL), parapneumonic effusion (689.9±413.6 U/mL), and transudates (all p<0.01) (Figure 1).
The pleural fluid/serum ratio of M30 was higher in tuberculosis (26.3±23.6) than in lung cancer (9.1±12.0), parapneumonic effusion (6.1±5.3), and transudates (2.5±1.5) (all p<0.01).
The M30 levels in pleural fluid were significantly correlated with the total cell count (rs=0.32, p<0.05), lymphocytic count (rs=0.52, p<0.05), total protein level (rs=0.45, p<0.05), and adenosine deaminase (ADA) level (rs=0.38, p<0.05) in pleural fluid (Figure 2).
In the ROC analysis of M30 in pleural fluid, the AUC (95% confidence interval) for differentiating tuberculous pleural effusion from all other effusions was 0.933 (0.852-0.977) for M30 and 0.949 (0.875-0.986) for ADA (Figure 3). The sensitivity and specificity of M30 for the diagnosis of tuberculous pleurisy compared to all other effusions were 82.1% and 84.3%, respectively, at a cut-off value of 1,233.8 U/mL. With regard to ADA, the sensitivity and specificity were 96.4% and 92.0%, respectively, at a cut-off value of 49.0 U/mL.
To determine additional diagnostic utility, subgroup analyses were conducted. When the ROC analysis was selectively done for differentiating tuberculous pleural effusion from parapneumonic effusion, M30 showed higher AUC than ADA (0.957 [0.847-0.995] vs. 0.902 [0.773-0.972]). When selectively analyzing the diagnostic performance of M30 for tuberculous pleural effusion cases with low ADA levels or minimal parenchymal lesions, the results did not show significant results.
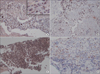
In the immunohistochemical analysis of M30, all pathologic types of cancer tissues showed moderate to high expression: squamous cell carcinoma (moderate, 2; high, 2), adenocarcinoma (moderate, 3; high, 1), and small cell carcinoma (moderate, 1; high, 3) (Figure 4). In subjects with tuberculous effusion, the epithelioid cells in the granulomas expressed M30 in the pleural tissues, and all cases showed high expression levels (Figure 5). The expression of M30 mostly showed perinuclear staining patterns. In parapneumonic effusion, all cases showed low expression of M30 in the pleural tissues (Figure 4).
This study showed that the level of M30 in pleural fluid, specifically that related to the activity of epithelial apoptosis, was increased in exudates and was more markedly increased in tuberculous pleural effusion. The M30 level in malignant pleural effusion was lower than that in tuberculous effusion, but higher than that in parapneumonic effusion. Immunohistochemical analyses showed that M30 was mainly expressed in malignant cells and epithelioid cells in the tuberculous granulomas. Tuberculous pleural effusion showed the strongest and most extensive expression of M30. The levels of M30 in pleural fluids were significantly higher than the levels in serum in all types of exudative pleural effusion. This suggests that epithelial apoptosis is locally activated in the milieu of pleural effusions and may play a role in the development of exudative pleural effusion.
Lung cancer mainly originates from respiratory epithelial cells, and higher levels of M30 in pleural fluid in malignant effusion may result from the activation of apoptosis in tumor cells due to enhanced cellular turnover12. In parapneumonic effusion, acute inflammation and tissue destruction are the main mechanisms of lung damage, and subsequent cell death is mainly due to necrosis rather than apoptosis23,24. This phenomenon may underlie the relatively lower levels of M30 in parapneumonic effusions than in lung cancer and tuberculosis. Among exudative pleural effusions, M30 levels were higher in tuberculous pleural effusion than in other pleural effusions. The higher levels than in malignant effusion were unexpected given that tuberculosis is characterized by chronic and indolent progression. With regard to the origin of M30 in tuberculous effusion, pulmonary epithelial cells may be its source. However, it is difficult to conclude that the prominent elevation in M30 levels in pleural fluids is mainly attributed to these cells. Furthermore, even cases with minimal or no pulmonary involvement showed similarly high levels of M30. Another candidate source may be epithelioid cells in granulomas. These cells in granulomas contain abundant cytokeratins in the cytoplasm25. To test this idea, we performed an immunohistochemical study of pleural tissues and found that epithelioid cells in granulomas highly expressed M30. Previous studies have also shown that epithelial cells mainly undergo necrosis while macrophage/epithelioid cells exhibit prominent apoptosis in tuberculosis25-27.
Although we speculate that the enhanced apoptosis of epithelioid cells in granulomas is the main source of cleaved cytokeratin 18, the exact pathogenic role of the apoptosis cannot be defined from the results of this study. There is no evidence that enhanced apoptosis of epithelioid cells plays a direct role in the development and progression of disease; rather, it could be a secondary phenomenon related to the active inflammatory response to infection with M. tuberculosis. This phenomenon may also underlie the relationship between the level of M30 in pleural fluids and the cellular and biochemical parameters in pleural effusions.
Although the diagnostic performance of M30 for tuberculous pleurisy cannot be expected to outperform well-known markers such as ADA and interferon-γ, it may play an adjunctive role, adding to the value of those markers. Furthermore, M30 showed a better discrimination between tuberculous pleural effusion and parapneumonic effusion than ADA. However, M30 failed to show a supplementary role for the diagnosis of tuberculous pleural effusion cases with low ADA levels and/or minimal parenchymal lesions.
In our immunohistochemical analyses, the expression of M30 was mainly localized in the perinuclear area. Kahn et al.28 reported that immunohistochemical staining for M30 in pleural fluids principally showed a concentric perinuclear ring pattern, and they found the same result with electron microscopic examination. Other immunohistochemical studies have also reported a perinuclear pattern in M30 expression during epithelial apoptosis29,30. This pattern may result from perinuclear aggregation of cleaved cytokeratins due to disruption of the cytoskeleton.
We acknowledge that there are limitations in this study. We included only a small number of cases. Therefore, to establish the clinical utility of M30 in pleural fluid in pleural effusion, further studies should be performed with more cases in the future. In the immunohistochemical analyses, full-scale quantitative scoring and comparison were not conducted because only representative cases were selectively studied. With regard to the demographics, patients with tuberculous effusion were younger and showed a slight female predominance. This may be a potential bias, although M30 levels according to sex and age did not reveal significant differences.
In conclusion, epithelial cell apoptosis was most prominently observed in tuberculous pleural effusion and showed clinical utility as a potential clinical marker. The main source of M30 may be the epithelioid cells of granulomas in tuberculous pleural tissues.
Figures and Tables
Figure 1
Comparison of M30 levels in serum (A) and pleural fluid (B) among patients with malignant pleural effusion, parapneumonic effusion, tuberculous pleural effusion, and transudate. The horizontal bar represents the median values. *p<0.01 compared to transudate group, **p<0.01 compared to all other groups.

Figure 2
Correlation of level of M30 in pleural fluids with total cell count (A), lymphocyte count (B), total protein level (C), and adenosine deaminase level (D).

Figure 3
Receiver operating characteristic analysis of M30 and adenosine deaminase (ADA) levels in pleural fluid for differentiating tuberculous pleural effusion from all other effusions.

Figure 4
Immunohistochemical staining for M30 in pleural tissues of lung cancer and parapneumonic effusion. M30 is mainly expressed in cancer cells of squamous cell carcinoma (A), adenocarcinoma (B), and small cell carcinoma (C). (D) Parapneumonic effusion showed weak expression. The inset in panel A shows the perinuclear staining pattern (A-D, ×200).
References
1. van den Berg E, van Woensel JB, Bem RA. Apoptosis in pneumovirus infection. Viruses. 2013; 5:406–422.
2. Zielinski RR, Eigl BJ, Chi KN. Targeting the apoptosis pathway in prostate cancer. Cancer J. 2013; 19:79–89.
3. Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002; 9:367–393.
4. Barrett KL, Willingham JM, Garvin AJ, Willingham MC. Advances in cytochemical methods for detection of apoptosis. J Histochem Cytochem. 2001; 49:821–832.
5. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010; 277:301–308.
6. Yuan Y, Liao YM, Hsueh CT, Mirshahidi HR. Novel targeted therapeutics: inhibitors of MDM2, ALK and PARP. J Hematol Oncol. 2011; 4:16.
7. Wu SH, Li CT, Lin CH, Chu JJ, Cheng ML, Lin KH. Soluble Fas ligand is another good diagnostic marker for tuberculous pleurisy. Diagn Microbiol Infect Dis. 2010; 68:395–400.
8. de Matos LL, Del Giglio AB, Matsubayashi CO, de Lima Farah M, Del Giglio A, da Silva Pinhal MA. Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn Pathol. 2012; 7:97.
9. Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013; 108:1526–1531.
10. Kawai M, Saegusa Y, Kemmochi S, Harada T, Shimamoto K, Shibutani M, et al. Cytokeratin 8/18 is a useful immunohistochemical marker for hepatocellular proliferative lesions in mice. J Vet Med Sci. 2010; 72:263–269.
11. De Petris L, Branden E, Herrmann R, Sanchez BC, Koyi H, Linderholm B, et al. Diagnostic and prognostic role of plasma levels of two forms of cytokeratin 18 in patients with non-small-cell lung cancer. Eur J Cancer. 2011; 47:131–137.
12. Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004; 214:1–9.
13. Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005; 59:Suppl 2. S359–S362.
14. Chung WY, Sun JS, Park JH, Lee HL, Lee KS, Kim YS, et al. Epithelial apoptosis as a clinical marker in idiopathic interstitial pneumonia. Respir Med. 2010; 104:1722–1728.
15. Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999; 187:567–572.
16. Roth GA, Krenn C, Brunner M, Moser B, Ploder M, Spittler A, et al. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope m30 in critically ill patients. Shock. 2004; 22:218–220.
17. Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009; 175:808–816.
18. Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007; 56:399–404.
19. Lee KS, Choi YH, Kim YS, Baik SH, Oh YJ, Sheen SS, et al. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med. 2008; 102:464–469.
20. Sikora J, Dworacki G, Zeromski J. Expression of Fas and Fas ligand and apoptosis in tumor-associated lymphocytes and in tumor cells from malignant pleural effusions. Nat Immun. 1998; 16:244–255.
21. Wang PS, Chen YM, Hsieh YL, Yu CF, Tsai CM, Perng RP. Pleural effusion and serum soluble fas-ligand levels are elevated in different clinical conditions. Lung. 2002; 180:25–32.
22. Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980; 69:507–512.
23. Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri P, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013; 17:e76–e83.
24. Zysk G, Bejo L, Schneider-Wald BK, Nau R, Heinz H. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin Exp Immunol. 2000; 122:61–66.
25. Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006; 74:6135–6144.
26. Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003; 5:649–660.
27. Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997; 65:298–304.
28. Kahn HJ, Hanna W, Yeger H, Baumal R. Immunohistochemical localization of prekeratin filaments in benign and malignant cells in effusions: comparison with intermediate filament distribution by electron microscopy. Am J Pathol. 1982; 109:206–214.
29. van Engeland M, Kuijpers HJ, Ramaekers FC, Reutelingsperger CP, Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp Cell Res. 1997; 235:421–430.
30. Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004; 297:11–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download