Abstract
Pulmonary artery sarcoma (PAS) is a rare, poorly differentiated malignancy arising from the intimal layer of the pulmonary artery. Contrast-enhanced chest computed tomography (CT) is a good diagnostic modality that shows a low-attenuation filling defect of the pulmonary artery in PAS patients. An 18-year-old man was referred to our hospital for the evaluation and management of cavitary pulmonary lesions that did not respond to treatment. A contrast-enhanced CT of the chest was performed, which showed a filling defect within the right interlobar pulmonary artery. The patient underwent a curative right pneumonectomy after confirmation of PAS. Although lung parenchymal lesions of PAS are generally nonspecific, it can be presented as cavities indicate pulmonary infarcts. Clinicians must consider the possibility of PAS as well as pulmonary thromboembolism in patients with pulmonary infarcts. So, we report the case with PAS that was diagnosed during the evaluation of cavitary pulmonary lesions and reviewed the literatures.
Pulmonary artery sarcoma (PAS) is a rare, poorly differentiated malignancy arising from the intimal layer of the pulmonary artery. The first published case was reported from an autopsy by Mandelstamm in 19231. Since then, only approximately 250 cases have been described, mostly as case reports2. PAS is not common, with an incidence of 0.001-0.03%, and hence, initial diagnosis is challenging3. Contrast-enhanced chest computed tomography (CT) is a good initial diagnostic modality that shows a low-attenuation filling defect of the pulmonary artery. However, PAS is frequently misdiagnosed as pulmonary thromboembolism, and further evaluations are needed for differential diagnosis. Lung parenchymal lesions shown by chest radiography or non-enhanced chest CT are mostly nonspecific4. Here, we report a case of an 18-year-old male patient with PAS who was diagnosed during evaluation of pulmonary cavitary lesions as seen on initial chest radiography and non-enhanced chest CT.
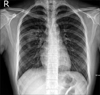
An 18-year-old man was referred to our institution from a primary clinic for further evaluation and management of cavitary pulmonary lesions. He had no significant medical history and had visited the primary clinic 3 months before he was referred to our institution. He presented with complaints of cough, blood-tinged sputum, and right chest wall pain. The initial chest radiography at the primary clinic showed patchy consolidations in the right upper lobe (Figure 1), and non-enhanced chest CT was performed. A 5-cm cavitary consolidation and multifocal nodular consolidations were observed in the right lung, mainly in the right upper lobe posterior segment and the right lower lobe (Figure 2A). The patient was treated with medication, including empirical antibiotics, and follow-up non-enhanced chest CT was performed every month for the next 3 months. Despite medical treatment, his symptoms did not improve and the lung parenchymal lesions persisted. Follow-up CT showed changes in the pulmonary lesions, decrease in the size of the main cavitary consolidation and increase in the number and size of adjacent nodular consolidations (Figure 2B-D). Eventually, the patient was referred to our institution for further diagnosis and treatment.
We suspected the possibility of various conditions that can cause cavitary lesions, including bacterial infection, fungal infection, tuberculosis, autoimmune disease, vasculitis, malignancy, and other rare diseases. The findings of laboratory examinations were unremarkable. The patient's leukocyte count was 7,870/µL with a C-reactive protein level of 0.44 mg/dL. We could not detect any microorganisms on the microbiologic studies including sputum culture, acid-fast bacilli stain, interferon-gamma release assay and aspergillous antigen test. The tests of autoantibodies such as antinuclear antibody and antineutrophil cytoplasmic antibody were negative. Further, no endobronchial lesion was observed on bronchoscopy. Percutaneous needle aspiration was performed, targeting the cavitary consolidation of the right upper lobe, but the pathologic results were unremarkable. Pathology examination showed parenchymal necrosis and fibrosis, interstitial lymphocytic infiltration, and type II pneumocyte hyperplasia. We did not detect granuloma, evidence of vasculitis, or any other possible causes of the pulmonary lesions.
Next, the patient underwent contrast-enhanced chest CT, which showed a filling defect within the lumen of the right interlobar pulmonary artery, raising the possibility of either pulmonary thromboembolism or PAS (Figure 3). The patient received low molecular weight heparin, considering the possibility of pulmonary thromboembolism. Deep vein thrombosis was not detected on venous duplex examination of the lower extremities. Positron emission tomography (PET)-CT with 18F-fluorodeoxyglucose (FDG) showed increased FDG uptake, with a maximum standardized uptake value of 9.6 along the right interlobar pulmonary artery, suggesting the presence of a malignant lesion (Figure 4).
To confirm the diagnosis, open thoracotomy and excisional biopsy were performed. At surgery, frozen biopsy specimens obtained from the right interlobar pulmonary artery revealed a PAS. Accordingly, the patient underwent subsequent curative right pneumonectomy.
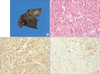
Grossly, a 1.5×1 cm gray-tan soft tumor was detected in the lumen of the right interlobar pulmonary artery (Figure 5A). The distal pulmonary artery was occluded with thrombus. A gray-tan-yellow infarct, measuring 4×2×2 cm, was observed in the right upper lobe, and multiple, small gray-tan nodules were scattered in the remaining lung.
Histopathologic examination of the intravascular tumor revealed abundant spindle cells with high cellularity, frequent mitoses, and nuclear pleomorphism (Figure 5B). Immunohistochemical staining was positive for smooth-muscle actin and desmin (Figure 5C, D). Staining for CD34 and CD56 was negative. Pathologic finding of the distal parenchymal lesions were ischemic necrosis and fibrosis, indicated pulmonary infarct. Also, there were hematogenous spread of occlusive tumor emboli and multiple parenchymal involvements within the right lung.
The patient recovered uneventfully and received 3 cycles of adjuvant chemotherapy with ifosfamide and doxorubicin. However, treatment was terminated because of side effects, which included severe gastrointestinal symptoms and neutropenia. There was no evidence of local recurrence or distant metastasis 6 months after surgery.
PAS is a rare tumor of the cardiovascular system, and very few cases have been reported in the literature. In most cases, PAS occurs in middle-aged patients, but cases of PAS in young patients, including a case of a 13-year-old child5, have also been reported. In our case, the patient was an adolescent, and therefore, it appears that PAS can occur in any age.
A variety of conditions is associated with pulmonary cavitary lesions. Infectious conditions include common bacterial infection; necrotizing pneumonia; lung abscess; septic emboli; pulmonary tuberculosis; fungal infection; non-tuberculosis mycobacterial infection; and other rare infections such as mucormycosis, actinomycosis, and cryptococcosis. Examples of non-infectious conditions are neoplasm, vasculitis such as Behcet's disease, Wegener's granulomatosis and pulmonary infarct due to pulmonary embolism or trauma6,7. In our case, the pulmonary cavitary lesion was eventually diagnosed as a pulmonary infarct due to PAS.
Because of its rarity and nonspecific clinical manifestations, PAS is often mistaken for pulmonary embolism, leading to inappropriate therapy such as prolonged anticoagulation or thrombolysis and thus delaying optimal therapy that might include surgery8. The absence of predisposing factors for pulmonary embolism (e.g., no deep vein thrombosis and no finding suggestive of a hypercoagulable state) and the lack of response to anticoagulation therapy are factors suggestive of PAS9. Lung parenchymal lesions observed on chest radiography or non-enhanced chest CT are generally nonspecific, but they sometimes indicate pulmonary infarcts10. On non-enhanced CT, an isodense filling defect can be detected. The contrast-enhanced CT features that suggest PAS rather than pulmonary embolism include a large low-density filling defect occupying the entire lumen of the pulmonary trunk or main branches of the pulmonary artery, with an increase in the diameter of the involved vessel and extraluminal extension of the tumor4,11. PET-CT is used to confirm the suspicion of PAS on the basis of increased radiopharmaceutical uptake by the tumor. Therefore, combining CT and PET-CT is very useful in assessing patients with suspected PAS12.
Patients with PAS have a poor prognosis, and treatment is rarely curative. If feasible, surgical resection, preferably performed with radical intent, is the treatment of choice. The role of chemotherapy and radiotherapy are still undefined. In 1995, the largest series to date, involving 6 cases, was published by researchers from the University of California. No patient survived longer than 19 months, even with adjuvant chemotherapy and radiation13. In contrast, in a case series and review of the literatures in 2009, researchers from MD Anderson Cancer Center showed encouraging results in 8 patients with PAS who underwent aggressive surgical resection and multimodality therapy2, reporting an estimated median survival time of 71 months and an estimated 5-year survival rate of 72.9%. On comparison of these results with those of 77 patients of previous studies (median survival time of 18±3.5 months and 5-year survival rate of 18.5%), the authors determined that the improved survival in their series might be attributable to two factors, aggressive resection with a curative intent and multimodality therapy. In addition, recently published case reports have emphasized the effectiveness of chemotherapy and multimodality therapy14. Further basic and clinical research on PAS is expected to establish the role of chemotherapy and radiotherapy and improve the outcome and survival of PAS15.
In our case, the diagnosis of PAS was delayed. Total 4 times of non-enhanced chest CT scans were performed in the primary clinic and the last one was performed just before the referral to our institution, so contrast-enhanced chest CT was performed later in the work-up of the patient. PAS is rare and is usually not considered in the differential diagnosis. However, early diagnosis is important because PAS has a dismal prognosis and surgical resection is the only curative therapeutic option available. Although PAS is very rare and parenchymal lesions are generally nonspecific, it can be presented as cavitary lesions indicate pulmonary infarcts. Clinicians must consider the possibility of PAS as well as pulmonary thromboembolism in patients with pulmonary infarcts.
Figures and Tables
Figure 2
Non-enhanced chest computed tomography (CT) images. (A) Initial non-enhanced chest CT shows a 5-cm cavitary consolidation in the right upper lobe posterior segment. (B-D) Follow-up CT show changes in the pulmonary lesions, decrease in size of the main cavitary consolidation and increase in the number and size of adjacent nodular consolidations.
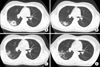
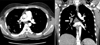
Figure 3
Contrast-enhanced chest computed tomography shows a filling defect within the lumen of the right interlobar pulmonary artery (arrows).
References
1. Mandelstamm M. Über primäre Neubildungen des Herzens. Virchows Arch Pathol Anat Physiol Klin Med. 1923; 245:43–54.
2. Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009; 87:977–984.
3. Mussot S, Ghigna MR, Mercier O, Fabre D, Fadel E, Le Cesne A, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg. 2013; 43:787–793.
4. Fukuda W, Morohashi S, Fukuda I. Intimal sarcoma of the pulmonary artery: diagnostic challenge. Acta Cardiol. 2011; 66:539–541.
5. Farooki ZQ, Chang CH, Jackson WL, Clapp SK, Hakimi M, Arciniegas E, et al. Primary pulmonary artery sarcoma in two children. Pediatr Cardiol. 1988; 9:243–251.
6. Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008; 21:305–333.
7. Kim NR, Han J. Pathologic review of cystic and cavitary lung diseases. Korean J Pathol. 2012; 46:407–414.
8. Delany SG, Doyle TC, Bunton RW, Hung NA, Joblin LU, Taylor DR. Pulmonary artery sarcoma mimicking pulmonary embolism. Chest. 1993; 103:1631–1633.
9. Mattoo A, Fedullo PF, Kapelanski D, Ilowite JS. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest. 2002; 122:745–747.
10. Ishiguro T, Kasahara K, Matsumoto I, Waseda R, Minato H, Kimura H, et al. Primary pulmonary artery sarcoma detected with a pulmonary infarction. Intern Med. 2007; 46:601–604.
11. Nagai T, Tabata H, Uehata A. Primary sarcoma of pulmonary artery resembling large pulmonary thrombus: diagnostic utility of different imaging modalities. Eur Heart J. 2012; 33:2271.
12. Ito K, Kubota K, Morooka M, Shida Y, Hasuo K, Endo H, et al. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009; 23:671–676.
13. Anderson MB, Kriett JM, Kapelanski DP, Tarazi R, Jamieson SW. Primary pulmonary artery sarcoma: a report of six cases. Ann Thorac Surg. 1995; 59:1487–1490.
14. Hoiczyk M, Iliodromitis K, Bauer S, Konorza T, Philipp S, Bankfalvi A, et al. Intimal sarcoma of the pulmonary artery with unusual findings: a case report. Clin Res Cardiol. 2012; 101:397–401.
15. Jamieson SW. Pulmonary artery sarcoma. Eur J Cardiothorac Surg. 2013; 43:793–794.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download