Abstract
Low-grade endometrial stromal sarcoma (ESS) is an uncommon gynecologic malignancy of mesodermal origin. Pulmonary metastasis of low-grade ESS can occur years and decades after the treatment of the primary disease. Low-grade ESS is frequently mistaken as benign uterine neoplasm like uterine leiomyoma, which can potentially lead to a misdiagnosis. We present a case of a 42-year-old woman with low-grade ESS, that initially presented as an incidental lung mass with multiple pulmonary nodules, seven years after an uterine myomectomy. A 6.9×5.8 cm-sized intrapelvic mass suspected of uterine origin was discovered while searching for potential extrathoracic primary origin. A pelviscopy and simultaneous thoracoscopic lung biopsy were conducted for pathologic diagnosis. Finally, the diagnosis was confirmed as low-grade ESS with lung metastasis based on the histopathologic examination with immunohistochemical stain, which was showed positive for CD10 and hormone receptor markers (estrogen and progesterone receptors) in both pelvic and lung specimens.
Metastatic lung tumors with extra-thoracic primary origin commonly presents as multiple pulmonary parenchymal nodules with various size at initial manifestation1. Metastatic neoplasm consists of the highest portion when incidental multiple lung nodules and mass are detected throughout a diagnostic chest radiography of computed tomography (CT) scan2.
When multiple incidental lung nodules or mass, suggestive of metastatic nature, are discovered, vigorous clinical evaluations to identify the primary site of origin are usually undergone2. If metastatic lung mass or nodules mimic radiologic findings of the primary lung cancer, or the primary malignancy shows an unusual presentation with a rare clinical incidence, diagnostic challenges occur occasionally. Additional troublesome arises when lung metastasis is manifested as a tumor recurrence, years and decades after the resection of the primary lesion.
In this article, we report a case with low-grade endometrial stromal sarcoma (ESS) who initially presented as an incidental lung mass with multiple pulmonary nodules, mimicking primary lung cancer, several years after myomectomy for a uterine leiomyoma.
A 42-year-old woman was referred to Korea University Ansan Hospital for evaluation of suspicious lung mass with multiple pulmonary nodules. The lung lesions were incidentally found in a simple chest roentgenography during a routine medical inspection performed a month ago. She had a history of myomectomy seven years ago in local clinic in China, and the pathologic result was known to be as a benign uterine tumor, leiomyoma.
At admission, she was asymptomatic and physical examination showed unremarkable findings. A chest X-ray revealed a right perihilar lung mass with tiny peripheral nodules on right middle lung field. Chest CT scan showed a 4.5-cm sized well demarcated right perihilar mass with various sized multiple peripheral nodules on both lungs (Figure 1). Her complete blood cell counts showed hemoglobin level of 14.0 g/dL, total leukocyte count of 7.50×109/L with 50.4% neutrophils and platelet count of 290×109/L. Blood biochemical results including blood urea nitrogen, creatinine, and liver function tests were unremarkable. Carcinoembryonic antigen, squamous cell carcinoma antigen, alpha-fetoprotein and beta human chorionic gonadotrophin also revealed values within normal range.
Initially, she was suspected of primary lung malignancy with lung to lung metastasis, but radiologic findings of the mass and nodules were well demarcated without remarkable lymph node enlargement in mediastinum and hilum. These features also suggested a possibility of metastatic lung tumor from extrathoracic origin. Thereby, locoregional and systemic evaluations were undertaken to discover potential extrathoracic metastatic lesions and concealed primary malignant origin. Because she had a history of surgical resection on leiomyoma, systemic evaluations to search possible primary origin included a gynecologic inspection on the preferential basis. Through pelvic examination by the gynecologist, firm, non-tender, smooth intrapelvic mass was palpated over the symphysis pubis, and transvaginal ultrasonography revealed a mixed echogenic mass sizing approximately 16-week pregnant uterus. Further radiologic evaluations were undergone to characterize detailed structural features of the intrapelvic mass.
Abdomen-pelvis CT scan discovered a 6.9×5.8-cm sized pelvic mass, and the mass showed a heterogeneous enhancement pattern with possible attachment to the adjacent intrapelvic structures. Magnetic resonance imaging was conducted for further delineation and characterization of the intrapelvic mass, and the lesion showed moderate enhancement in T1 weighted image with internal fibrovascular septum (Figure 2). The mass was partially abutting to the uterine fundus in a possible outgrowing fashion. Initial radiologic impression of the intrapelvic mass was neoplasm from uterine origin including subserosal myoma or fibrous tumor such as malignant fibrous histocytoma. In positron emission tomography-CT scan, neither pulmonary lesions nor pelvic mass expressed hypermetabolisms typical of malignancy (Figure 3). Maximum standardized uptake values of the intrapelvic mass and the right perihilar mass were 1.32 and 1.02, respectively. This finding was generally discordant with the initial impressions suggesting either primary pulmonary or metastatic malignancy. Furthermore, this feature potentially implied that both lung and pelvic mass were mutually sharing identical characteristics. Therefore, pathologic evaluation on both lung and pelvic mass was planned to confirm the diagnosis.
Bronchoscopic examination for the right perihilar mass showed no definite endobronchial lesion. Percutaneous needle biopsy for the right perihilar mass could not be performed because the location of the mass was just beside the right pulmonary artery and its branches. Therefore, surgical biopsy under general anesthesia was decided for the simultaneous pathologic diagnosis of both pelvic and lung mass.
Diagnostic pelviscopy for intrapelvic mass and thoracoscopic right perihilar mass biopsy with wedge resection for right peripheral nodules were conducted. Intraoperative frozen biopsies showed small round malignant cells from pelvic and lung specimens, which was not compatible for typical non-small cell lung cancer. Intraoperative gross finding of the pelvic mass exhibited bulging appearance from the anterior side of the uterine fundus, and this suggested higher probability of uterine origin malignancy. Subsequently, diagnostic pelviscopy was converted into total abdominal hysterectomy with both salphingo-oophorectomy.
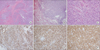
Histopathologic examination for specimens from lung mass, uterus, both ovary and salpinx, and omentum showed same features to each other (Figure 4A-C). Bland looking small oval to spindle shaped cells with scanty cytoplasm and perivascular arrangement were observed on hematolxyline and eosin stain. These cells were infiltrating adjacent normal myometrium and pulmonary parenchymal structures. Immunohistochemical stain of the uterine specimen revealed positivites on estrogen and progesterone receptors, and CD10 (Figure 4D, E). However, cytokeratin, chromogranin, smooth muscle actin, and p53 were negative. These immunohistochemical features, especially positive to CD10, were identical to the findings of the right perihilar lung mass (Figure 4F). Based on these histopathological and immunohistochemical findings, the diagnosis of low-grade ESS with pulmonary metastasis was confirmed.
After recovery from post-operative period, palliative chemotherapy with paclitaxel, adriamycin and cisplatin was initiated. Abdomen-pelvis CT scan after two cycles of chemotherapy showed no significant evidence of recurrence in the pelvic cavity. From the chest CT scan taken at this point of time, remnant right perihilar mass remained to be unchanged without any sign of disease progression, and there was no newly developed pulmonary nodule suggestive of metastasis.
Uterine sarcomas are uncommon gynecologic malignancy of mesodermal origin which constitute 2-6% of all uterine malignancies, and account for 1% of female genital tract malignancies3,4. ESS represents approximately 15-25% of all uterine sarcomas and annual incidence of ESS is reported around 2 cases per million women3,5.
Histologically, endometrial stromal neoplasms are divided into three groups depending upon mitotic activity, vascular invasion and prognostic properties: endometrial stromal nodule, low-grade endometrial sarcoma, high-grade or undifferentiated ESS6,7. Low-grade ESS is the most common pathologic subtype which consists approximately 80% of stromal neoplasm, and usually presented as slow-growing neoplasm with an indolent clinical course8,9. Prognosis of low-grade ESS showed an excellent clinical outcome up to survival rate of 100% in 5- to 10-year follow-up data7,10. Under proper management with long term progestin maintenance therapy or with other proposed adjunctive therapeutic modalities, presence of extra-pelvic metastatic lesions such as pulmonary nodules seems to have minimal effect on overall survival5,10.
Manifestation of pulmonary metastasis of low-grade ESS shows a wide clinical spectrum from an asymptomatic presentation, as like in the current case, to accompanying various respiratory symptoms. Most common respiratory symptoms were dyspnea, chest pain and cough in the previous reports, and extreme manifestation such as a bilateral pneumothorax has been also reported10. Pulmonary metastasis of low-grade ESS develops less frequently, however lung is the most common site of distant metastasis, reporting in a range from 7% to 30% in the previous studies8,10.
Diagnostic challenges develop especially in the clinical setting of recurrent disease of the low-grade ESS after the treatment of the primary lesion. Recurrence of the disease is reported to develop in one third to one half of patients after treating the primary lesion9. Recurrence can even appear 30 years after the treatment mostly as local recurrence5. Other cases often present as extra-pelvic metastasis, for instance, multiple lung metastasis or peritoneal metastasis11.
If the recurrence of extra-thoracic neoplasm as multiple pulmonary nodules or masses is suspected, previous pathology report or surgical specimens of the extra-thoracic neoplasm must be reviewed. In available cases, this piece of information often could be a crucial cornerstone for differential diagnosis against the primary lung malignancies. Review on the previous gynecologic specimens should be strongly emphasized when unusual mesenchymal lung neoplasms are encountered in the female population10. When recurrent disease of low-grade ESS manifests solely as pulmonary metastasis, misdiagnosis can occur unless clinicians and/or pathologists are aware of the prior history of intra-pelvic neoplasm12. Potential misdiagnosis of the prior uterine neoplasm, which is suspected in our case, can be also misleading to the wrong clinical impressions10. Based on the previous history of uterine leiomyoma, other benign conditions resembling the features of pulmonary metastasis, such as benign metastasizing leiomyoma, also could be a possible clinical diagnosis before the pathologic confirmation13.
Recently, immunohistochemical markers such as CD10 could provide further crucial leads to the differential diagnosis of low-grade ESS to other uterine smooth muscle neoplasms during the pathologic evaluation14. CD10 is an enzymatic regulator of local peptide concentration which modulates cellular response to peptide hormones15. Various hormone-sensitive and peptide-sensitive cells, both normal cell and neoplasm, express CD10 antigen, and this includes low-grade ESS and its normal counterpart14. Although CD10 is expressed in various nonhematopoietic neoplasms, among the immunohistochemical profiles for uterine sarcomas, CD10 is currently the most specific marker for differentiating ESS.
When metastatic lung malignancies are clinically suspected in the presence of multiple lung nodules and/or masses, close considerations to patient's previous medical history and underlying comorbidities should be made for a better diagnostic and therapeutic guidance. History of gynecologic neoplasm, even a previously reported benign neoplasm, should not be overlooked when metastatic lung malignancies are suspected.
Figures and Tables
Figure 1
(A, B) Chest computed tomography revealed a 4.5-cm sized well demarcated right perihilar mass (arrowhead) with mild enhancement of soft tissue density. Various sized circumscribed multiple nodules (arrows) are observed in periphery of both lungs.

Figure 2
Pelvic magnetic resonance imaging scan showed a 6.9×5.8-cm sized mass with internal fibrovascular septum (arrow) in midpelvic cavity which was moderately enhanced in a T1 weighted image (A). T2 weighted image (B) showed high signal intensity of the mass which was slightly abutted to the uterine fundus (arrowhead) suggestive of uterine origin rather than adnexal origin.
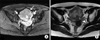
Figure 3
In positron emission tomography-computed tomography scan, pelvic mass (A) showed minimal hypermetabolism with maximum standardized uptake value of 1.32. Right perihilar mass (B) showed no definite fluorodeoxyglucose uptake, with maximum a standardized uptake value of 1.02.
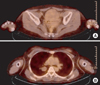
Figure 4
Histopathologic examinations showed innocuous looking small oval to spindle shaped cells with scanty cytoplasm (C, H&E, stain, ×400) and perivascular arrangement which were infiltrating the normal myometrium (A, H&E stain, ×40) and lung structure (B, H&E stain, ×40). In immunohistochemical stain, tumor cells of uterus were strongly positive to progesterone receptors (D, ×200) and CD10 (E, ×200). Lung mass also showed positive CD10 staining (F, ×200).
References
1. Crow J, Slavin G, Kreel L. Pulmonary metastasis: a pathologic and radiologic study. Cancer. 1981; 47:2595–2602.
2. Gross BH, Glazer GM, Bookstein FL. Multiple pulmonary nodules detected by computed tomography: diagnostic implications. J Comput Assist Tomogr. 1985; 9:880–885.
3. D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010; 116:131–139.
4. Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986; 76:399–402.
5. Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003; 90:170–176.
6. Haberal A, Kayikcioglu F, Boran N, Caliskan E, Ozgul N, Kose MF. Endometrial stromal sarcoma of the uterus: analysis of 25 patients. Eur J Obstet Gynecol Reprod Biol. 2003; 109:209–213.
7. Norris HJ, Taylor HB. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966; 19:755–766.
8. Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms: a clinicopathologic study of 117 cases. Am J Surg Pathol. 1990; 14:415–438.
9. Garavaglia E, Pella F, Montoli S, Voci C, Taccagni G, Mangili G. Treatment of recurrent or metastatic low-grade endometrial stromal sarcoma: three case reports. Int J Gynecol Cancer. 2010; 20:1197–1200.
10. Aubry MC, Myers JL, Colby TV, Leslie KO, Tazelaar HD. Endometrial stromal sarcoma metastatic to the lung: a detailed analysis of 16 patients. Am J Surg Pathol. 2002; 26:440–449.
11. Pink D, Lindner T, Mrozek A, Kretzschmar A, Thuss-Patience PC, Dorken B, et al. Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol. 2006; 101:464–469.
12. Abrams J, Talcott J, Corson JM. Pulmonary metastases in patients with low-grade endometrial stromal sarcoma. Clinicopathologic findings with immunohistochemical characterization. Am J Surg Pathol. 1989; 13:133–140.
13. Fu Y, Li H, Tian B, Hu B. Pulmonary benign metastasizing leiomyoma: a case report and review of the literature. World J Surg Oncol. 2012; 10:268.
14. Chu PG, Arber DA, Weiss LM, Chang KL. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001; 14:465–471.
15. Shipp MA, Stefano GB, D'Adamio L, Switzer SN, Howard FD, Sinisterra J, et al. Downregulation of enkephalin-mediated inflammatory responses by CD10/neutral endopeptidase 24.11. Nature. 1990; 347:394–396.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download