Abstract
Background
We investigated whether wogonin and apigenin significantly affect the epidermal growth factor receptor (EGFR) signaling pathway involved in MUC5AC mucin gene expression, and production from cultured airway epithelial cells; this was based on our previous report that apigenin and wogonin suppressed MUC5AC mucin gene expression and production from human airway epithelial cells.
Methods
Confluent NCI-H292 cells were pretreated with wogonin or apigenin for 15 minutes or 24 hours and then stimulated with epidermal growth factor (EGF) for 24 hours or the indicated periods.
Pulmonary mucus plays a pivotal role in defensive action against invading pathogenic microorganisms, chemicals and particles. Mucins are high molecular weight glycoproteins present in the airway mucus and produced by goblet cells in the surface epithelium as well as mucous cells in the submucosal gland. However, hypersecretion of airway mucus is one of the major symptoms associated with severe pulmonary diseases including asthma, chronic bronchitis, cystic fibrosis and bronchiectasis1,2. Therefore, we suggest it is valuable to find the potential activity of regulating the excessive mucin production and/or secretion by the compounds derived from various medicinal plants. Flavonoids are a group of phenolic phytochemicals, common in various medicinal plants, vegetables and fruits3. We have tried to investigate the possible activities of some flavonoids on mucin production (secretion) from airway epithelial cells. As a result of our trial, we previously reported that several compounds affected mucin production and/or secretion from airway epithelial cells4,5,6,7. According to folk medicine, Scutellaria baicalensis Georgi has been used for regulating airway allergic and inflammatory diseases8. Also, two of their components-wogonin and apigenin-were reported to have various biological effects including antiinflammatory effect9,10,11,12,13,14,15. In our previous study, wogonin inhibited tumor necrosis factor α-induced MUC5AC mucin gene expression and production through the inactivation of nuclear factor-κB signaling in airway epithelial cells16. Also, we demonstrated that wogonin or apigenin inhibited epidermal growth factor (EGF)- or phorbol myristate acetate-induced MUC5AC mucin production and gene expression17. Expression of epidermal growth factor receptor (EGFR) is increased in asthmatic human airways18. EGF stimulates MUC5AC mucin gene expression and protein synthesis via binding to EGFR in a human airway epithelial cell line19. However, to the best of our knowledge, no other studies on these two compounds-wogonin and apigenin-on EGFR signaling pathway involved in MUC5AC mucin gene expression and production in human airway epithelial cells have been carried out. Therefore, in this study, we checked whether wogonin or apigenin affects the phosphorylation of EGFR, MEK1/2 and ERK1/2 in NCI-H292 cells, a human pulmonary mucoepidermoid cell line, which are frequently used for the purpose of studying the signaling pathway involved in airway mucin production and gene expression20,21,22.
All the chemicals and reagents used in this study including wogonin (purity: 98.0%) and apigenin (purity: 95.0%) were purchased from Sigma (St. Louis, MO, USA) unless otherwise specified. Anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). EGFR, p-EGFR, p-MEK1/2, and p-ERK1/2 antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 units/mL), streptomycin (100 µg/mL), and HEPES (25 mM) at 37℃ in a humidified 5% CO2, 95% air water-jacketed incubator. For serum deprivation, at 60% confluence, cultures were washed twice with phosphate-buffered saline (PBS) and recultured in RPMI 1640 with 0.2% FBS for 24 hours.
NCI-H292 cells were seeded at a density of 2×105/mL (0.1 mL/well) in 96-well microtiter plates and allowed to attach for 24 hours to keep the log phase growth at the time of drug treatment. Wogonin or apigenin was dissolved in dimethylsulfoxide (DMSO) and administered in RPMI 1640 supplemented with 10% FBS in the presence of penicillin (100 units/mL), streptomycin (100 µg/mL), and HEPES (25 mM) (final concentrations of DMSO were under 0.5%). 0.5% DMSO alone did not affect the proliferation of cells. After incubation with the indicated drug concentrations for 72 hours, cell proliferation (viability) was determined using the sulforhodamine B (SRB) assay.
For measurement of mucin production, after 24 hours of serum deprivation, NCI-H292 cells were pretreated with varying concentrations of wogonin or apigenin for 30 minutes and treated with EGF (25 ng/mL) for 24 hours in serum-free RPMI 1640. Wogonin or apigenin was dissolved in DMSO and treated in culture medium (final concentrations of DMSO were 0.5%). The final pH values of these solutions were between 7.0 and 7.4. Culture medium and 0.5% DMSO did not affect mucin production from NCI-H292 cells. After 24 hours, cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and collected to measure the production of MUC5AC protein (in 24-well culture plate). For western blot analysis, NCI-H292 cells (confluent in 150 mm culture dish) were pretreated with 20 µM of wogonin or apigenin for 15 minutes or 24 hours and treated with EGF (25 ng/mL) for 24 hours or the indicated periods in serum-free RPMI 1640. Wogonin or apigenin was dissolved in DMSO and treated in culture medium (final concentrations of DMSO were 0.5%). Culture medium and 0.5% DMSO did not affect the phosphorylations of EGFR, MEK1/2, and ERK1/2 in NCI-H292 cells.
MUC5AC protein was measured by using enzyme-linked immunosorbent assay. Cell lysates were prepared with PBS at 1:10 dilution, and 100 µL of each sample was incubated at 42℃ in a 96-well plate, until being dry. Plates were washed three times with PBS and blocked with 2% bovine serum albumin for 1 hour at room temperature. Plates were again washed three times with PBS and then incubated with 100 µL of 45M1, a mouse monoclonal MUC5AC antibody (1:200, NeoMarkers, Fremont, CA, USA) which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 hour, the wells were washed three times with PBS, and 100 µL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 hour, plates were washed three times with PBS. Color reaction was developed with 3,3',5,5'-tetramethylbenzidine (TMB) peroxide solution and stopped with 1 N H2SO4. Absorbance was read at 450 nm.
After the treatment, media were aspirated and the cells were washed with cold PBS. The cells were collected by scraping and centrifuged in a microcentrifuge (3,000 rpm, 5 minutes, 4℃). The supernatant was discarded. The cells were mixed with RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) for 30 minutes with continuous agitation. The lysate was centrifuged in a microcentrifuge at 14,000 rpm for 15 minutes at 4℃. The supernatant was used immediately or stored at -80℃. Protein content in extract was determined by Bradford method.
Whole cell extracts containing proteins (each 20-60 µg as protein) were subjected to 7-15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto the PVDF membrane. The blots were blocked using 5% skim milk and probed with appropriate primary antibody in blocking buffer overnight at 4℃. The membrane was washed with PBS and then probed with the secondary antibody conjugated with horseradish peroxidase (Calbiochem, San Diego, CA, USA). Immunoreactive bands were detected by an enhanced chemiluminescence kit (Pierce ECL western blotting substrate; Thermo Scientific, Waltham, MA, USA).
As can be seen in Figure 1, wogonin or apigenin did not affect the cell proliferation at the concentrations of 5, 10, and 20 µM. However, 50 and 100 µM of wogonin or apigenin significantly inhibited the proliferation of NCI-H292 cells. The numbers of cells in wogonin-treated cultures were 100±13%, 96±14%, 91±18%, 98±12%, 61±9%, and 52±6% for control, 5, 10, 20, 50, and 100 µM, respectively. The numbers of cells in apigenin-treated cultures were 100±9%, 89±12%, 95±10%, 93±9%, 58±11%, and 58±8% for control, 5, 10, 20, 50, and 100 µM respectively.
As can be seen in Figure 2, wogonin significantly inhibited EGF-induced MUC5AC production from NCI-H292 cell. The amounts of mucin in the cells of wogonin-treated cultures were 100±13%, 286±23%, 195±13%, 179±7%, and 101±12% for control, 25 ng/mL of EGF alone, EGF plus wogonin 5 µM, EGF plus wogonin 10 µM and EGF plus wogonin 20 µM, respectively. The amounts of mucin in the cells of apigenin-treated cultures were 100±13%, 286±23%, 201±16%, 162±8%, and 93±6% for control, 25 ng/mL of EGF alone, EGF plus apigenin 5 µM, EGF plus apigenin 10 µM and EGF plus apigenin 20 µM respectively.
EGF (25 ng/mL, 24 hours) augmented the expression and phosphorylation of the EGFR. On the other hand, apigenin or wogonin significantly inhibited this expression and phosphorylation of EGFR, as shown by western blot analysis with the corresponding specific antibodies (Figure 3).
EGF increased the phosphorylation of MEK1/2 in NCI-H292 cells. However, pretreatment of wogonin or apigenin decreased the phosphorylation of MEK1/2 (Figure 4).
EGF increased the phosphorylation of p44/42 mitogen-activated protein kinase (MAPK) in NCI-H292 cells. However, pretreatment of wogonin or apigenin decreased the phosphorylation of p44/42 MAPK (Figure 5).
As aforementioned in introduction, we demonstrated in our previous study that wogonin or apigenin inhibited EGF-induced MUC5AC mucin protein production and gene expression17. In the present study, we tried again to clarify the effects of wogonin and apigenin on production of airway MUC5AC mucin and got the same result (Figure 2). Based on this result, we investigated whether wogonin or apigenin at the concentration of 20 µM affects the EGFR signaling cascade. EGF activates EGFR signaling cascade and MUC5AC expression in NCI-H292 cells. EGFR serves a central role as a primary regulator of epithelial function, transducing extracellular signals from its activating ligands into intracellular signaling cascades through dimerization and transphosphorylation catalyzed by the intrinsic tyrosine kinase23. To induce EGFR activation, two different pathways have been reported. First, the binding of its ligands to EGFR activates the intrinsic receptor tyrosine kinase and induces tyrosine phosphorylation24. Second, tyrosine phosphorylation of EGFR can be activated by a ligand-independent mechanism called transactivation. EGFR transactivation is known to occur with various stimuli such as H2O225, ultraviolet light, osmotic stress26, and growth hormone27. Depending on the triggering stimuli, the signal transduction pathways which control mucin transcription have distinct pathways1,28. EGFR activation also plays a major role in modulating mucus production in airway epithelium. In this study, EGF, an endogenous ligand of the EGFR, was selected as a direct activator of this pathway based on previous studies in cultured human airway epithelial NCI-H292 cells21,29. It was found that EGFR is constitutively expressed in NCI-H292 cells, as faint band observed in western blot analysis with anti-EGFR monoclonal antibody in the control group (Figure 3). Being consistent with the notion that the overexpression of MUC5AC is the consequence of the activation of the EGFR signaling cascade, Mata et al.30 also found that preincubation with EGFR tyrosine kinase inhibitors prevented the EGF-induced augmentation of the MUC5AC gene expression and protein production. EGF increases the protein tyrosine kinase activity of its receptor and thereby activates other kinase cascades such as MAPKs including p38 and p44/42 MAPKs31. As can be seen in results, EGF (25 ng/mL, 24 hours) augmented the expression and phosphorylation of the EGFR and 20 µM of wogonin or apigenin significantly inhibited this expression and phosphorylation (Figure 3). There was no cytotoxic effect of wogonin or apigenin at the concentration of 20 µM, based on the data from cell viability test using SRB assay (Figure 1). Also, EGF increased the phosphorylation of MEK1/2 in NCI-H292 cells. However, pretreatment of wogonin or apigenin decreased the phosphorylation of MEK1/2 (Figure 4). As expected, it was found that an early activation of p44/42 MAPK was observed as well as phosphorylation of EGFR after exposure to EGF for 24 hours (Figure 5). Mata et al.30 reported that inhibition of p38 and p44/42 MAPKs with the selective inhibitors SB20202190 and PD98059 abrogated the EGF-induced MUC5AC gene expression. In our result, EGF increased the phosphorylation of p44/42 MAPK in NCI-H292 cells. However, pretreatment of wogonin or apigenin decreased the phosphorylation of p44/42 MAPK (Figure 5). Taken together, the result from this study suggests a possibility of using wogonin or apigenin as a new efficacious mucoregulator for inflammatory pulmonary diseases, although further studies are essential.
In conclusion, Wogonin or apigenin inhibits EGFR signaling pathways, which may explain how they inhibit MUC5AC mucin gene expression and production induced by EGF.
Figures and Tables
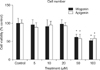 | Figure 1Effect of wogonin or apigenin on proliferation of NCI-H292 cells. NCI-H292 cells were incubated for 72 hours in the presence of varying concentrations of wogonin or apigenin. Cell viability was determined using the sulforhodamine B assay as described in Materials and Methods. Each bar represents a mean±standard error of mean of three independent experiments in comparison with the control set at 100%. *Significantly different from control (p<0.05). |
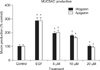 | Figure 2Effect of wogonin or apigenin on epidermal growth factor (EGF)-induced MUC5AC mucin production from NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations of wogonin or apigenin for 30 minutes and then stimulated with EGF (25 ng/mL) for 24 hours. Cell lysates were collected for measurement of MUC5AC mucin production by enzyme-linked immunosorbent assay. Three independent experiments were performed and the representative data were shown. Each bar represents a mean±standard error of mean of 3 culture wells in comparison with the control set at 100%. *Significantly different from control (p<0.05). †Significantly different from EGF alone (p<0.05). |
 | Figure 3Effect of wogonin or apigenin on epidermal growth factor receptor (EGFR) expression and phosphorylation. NCI-H292 cells were pretreated with wogonin (20 µM) or apigenin (20 µM) for 15 minutes and then stimulated with epidermal growth factor (EGF; 25 ng/mL) for 24 hours. Whole cell extract was collected and western blot analysis of the cellular proteins with anti-EGFR and anti-phospho-EGFR (Tyr1068) antibodies was conducted. Three independent experiments were performed and the representative data were shown. |
 | Figure 4Effect of wogonin or apigenin on phosphorylation of MEK1/2. NCI-H292 cells were pretreated with wogonin (20 µM) or apigenin (20 µM) for 24 hours and then stimulated with epidermal growth factor (EGF; 25 ng/mL) for the indicated periods. Whole cell extract was collected and western blot analysis of the cellular proteins with anti-phospho MEK1/2 (Ser221) antibody was conducted. Three independent experiments were performed and the representative data were shown. |
 | Figure 5Effect of wogonin or apigenin on phosphorylation of p44/42. NCI-H292 cells were pretreated with wogonin (20 µM) or apigenin (20 µM) for 24 hours and then stimulated with epidermal growth factor (EGF; 25 ng/mL) for the indicated periods. Whole cell extract was collected and western blot analysis of the cellular proteins with anti-phospho p44/42 (Thr202/Tyr204) antibody was conducted. Three independent experiments were performed and the representative data were shown. |
Acknowledgements
This research was supported by a grant (12172KFDA989) from Korea Food & Drug Administration in 2012.
References
1. Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999; 160(5 Pt 2):S44–S48.
2. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.
3. Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002; 102:1414–1420.
4. Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, et al. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine. 2011; 18:743–747.
5. Lee HJ, Lee SY, Jeon BK, Lee JW, Kim YS, Lee MN, et al. Effect of platycodin D on airway MUC5AC mucin production and gene expression induced by growth factor and proinflammatory factor. Biomol Ther. 2010; 18:294–299.
6. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011; 25:1196–1200.
7. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009; 23:1458–1461.
8. Jang IM. Treatise on Asian herbal medicines. Seoul: Haksul-Pyunsukwan in Research Institute of Natural Products of Seoul National University;2003.
9. Clere N, Faure S, Martinez MC, Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem. 2011; 9:62–77.
10. Paoletti T, Fallarini S, Gugliesi F, Minassi A, Appendino G, Lombardi G. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur J Pharmacol. 2009; 620:120–130.
11. Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, et al. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur J Cancer. 2010; 46:3365–3374.
12. Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol Pharmacol. 2011; 79:886–893.
13. Li RR, Pang LL, Du Q, Shi Y, Dai WJ, Yin KS. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol Immunotoxicol. 2010; 32:364–370.
14. Cho J, Lee HK. Wogonin inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Biol Pharm Bull. 2004; 27:1561–1564.
15. Chun W, Lee HJ, Kong PJ, Lee GH, Cheong IY, Park H, et al. Synthetic wogonin derivatives suppress lipopolysaccharide-induced nitric oxide production and hydrogen peroxide-induced cytotoxicity. Arch Pharm Res. 2005; 28:216–219.
16. Sikder MA, Lee HJ, Mia MZ, Park SH, Ryu J, Kim JH, et al. Inhibition of TNF-alpha-induced MUC5AC mucin gene expression and production by wogonin through the inactivation of NF-kappaB signaling in airway epithelial cells. Phytother Res. 2014; 28:62–68.
17. Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, et al. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012; 26:1784–1788.
18. Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998; 157(6 Pt 1):1907–1912.
19. Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004; 59:992–996.
20. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972.
21. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.
22. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.
23. Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994; 19:459–463.
24. Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981; 24:741–752.
25. Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, et al. EGF-Receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998; 19:786–798.
26. Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996; 274:1194–1197.
27. Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, et al. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997; 390:91–96.
28. Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN, et al. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 2002; 26:558–564.
29. Kohri K, Ueki IF, Shim JJ, Burgel PR, Oh YM, Tam DC, et al. Pseudomonas aeruginosa induces MUC5AC production via epidermal growth factor receptor. Eur Respir J. 2002; 20:1263–1270.
30. Mata M, Sarria B, Buenestado A, Cortijo J, Cerda M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005; 60:144–152.
31. Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003; 4:651–657.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download