Abstract
Urothelial carcinomas (UCs) can occur in the upper urinary tract or lower urinary tract. Upper urinary tract urothelial carcinoma (UUT-UC) is relatively a rare disease and accounts for only about 5% of UC cases. Sporadic cases of late-onset metastasis, associated with UC of the bladder, have occasionally been reported. In contrast, no late-onset distant metastatic UUT-UC without local recurrence has, to the best of our knowledge, been reported in the English literature. We report an extremely rare case of distant metastatic UC, mimicking lung adenocarcinoma that originated from UUT-UC 12 years previously.
Urothelial carcinomas (UCs) can occur in the upper urinary tract (pyelocaliceal cavities and ureters) or lower urinary tract (bladder and urethra). Bladder tumors account for 90-95% of UCs1. However, upper urinary tract urothelial carcinoma (UUT-UC) is relatively uncommon and accounts for only about 5% of UC cases2. While there have been sporadic case reports of distant metastasis later in life associated with UC of the bladder3,4, distant metastasis without local recurrence 12 years after diagnosis of UUT-UC has not, to the best of our knowledge, been described in the literature. In this paper, we present an extremely rare case of distant metastatic UC mimicking lung adenocarcinoma originating from UUT-UC 12 years previously.
A 50-year-old man was referred to our hospital with presumed lung cancer. He reported blood-tinged sputum for 5 days but was otherwise well. He had a 40-pack-year history of cigarette smoking. Twelve years earlier, after two episodes of gross hematuria, a diagnosis of UC of the renal pelvis had been made at our hospital. Right nephroureterectomy with bladder cuff excision was performed. Pathological examination revealed a UC in the renal pelvis (T1N0M0, grade 2) (Figure 1A), and a tumor involving the ureteral margin. The patient underwent adjuvant chemotherapy (methotrexate, vinblastine, doxorubicin, and cisplatin: three cycles for 3 months). Thereafter, he was followed up through regular cystoscopy and/or abdominal computed tomography (CT) at the urology clinic for 10 years, and no additional tumors were identified.
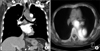
At admission, the serum level of carcinoembryonic antigen was 9.73 ng/mL (reference range, 0.0-6.0 ng/mL). Other laboratory test results were normal. A chest CT scan showed a mass lesion in the left lower lobe of the lung and lymphadenopathy in the left lower paratracheal and subcarinal areas. Bronchoscopic examination revealed no endobronchial lesions. A CT-guided fine-needle biopsy of lung demonstrated histologic features suggestive of adenocarcinoma (Figure 1B). No other primary lesions were found by 18F-fluorodeoxyglucose positron emission tomography/computed tomography, digestive endoscopy, abdominal CT or urine cytology. Brain magnetic resonance imaging revealed brain metastases. Therefore, we diagnosed the patient with advanced lung adenocarcinoma. After brain CyberKnife radiosurgery, he underwent two cycles of cisplatin-based combination chemotherapy, which had no effect. After chemotherapy, a small nodule in the mid-trachea was noted on bronchoscopic examination. Pathological analysis of a bronchoscopic biopsy showed a poorly differentiated carcinoma (Figure 1C). Immunohistochemical analysis showed the tumor cells to be positive for cytokeratin 7 and p63 (Figure 2), and negative for thyroid transcription factor 1 (TTF-1). These findings suggested possible metastatic UC. All histopathological samples, including the previous UC of the renal pelvis (Figure 1A), CT-guided fine-needle biopsy of the lung (Figure 1B), and tracheal biopsy (Figure 1C), were reviewed. The results of pathologic analysis revealed a high-grade metastatic UC following a previous low-grade UC of the renal pelvis. Systemic re-evaluation revealed concomitant aggravation of lung, esophagus, and bone metastases (Figure 3). In spite of radiotherapy and supportive care, the patient died as a result of aggravation of multiple metastasis.
UUT-UC is a relatively uncommon disease, accounting for about 5% of all UC cases and 5-10% of all renal tumors2,5. Because of its rarity, useful information can only be based on an extensive literature review1,2,5-8.
While the incidence of renal pelvic tumors has remained fairly constant over the last 30 years, the incidence of ureteral tumors has increased slightly. Fortunately, disease-specific survival has improved slightly7. Generally, UUT-UC rarely occurs before the age of 40. Its incidence peaks in the fifties, sixties and seventies. Nephroureterectomy with bladder cuff removal remains the standard treatment for UUT-UC1,2. In the largest retrospective study of UUT-UC5, the median time to recurrence was 12.0 months (mean, 23.6 months; range, 1-99 months). Common sites of distant metastases include the lungs, bone and the liver. Tumor stage and treatment modality were shown to be predictors of disease recurrence in a multivariate analysis. Another study of UC of the bladder showed that brain metastases occurred in fewer than 1% of patients with UC and that 37% of patients had metastatic disease with no bladder or pelvic recurrence6. Sporadic cases of late-onset metastasis associated with UC of the bladder have occasionally been reported3,4. To the best of our knowledge, this is the first case of distant metastatic UC without local recurrence 12 years after diagnosis of UUT-UC.
In recent years, as a result of increasing experience with UC, the spectrum of microscopic forms of UC has been expanded to include several unusual histological variants. The recognition of histological variants of UC is important because some types may be associated with a different clinical outcome or have a different therapeutic approach. Moreover, awareness of the unusual pattern may be critical in avoiding diagnostic misinterpretation. Furthermore, some of UCs with glandular differentiation have similar histology to adenocarcinoma9,10. Therefore, immunohistochemical studies can be useful in the identification of histological variants of UCs. To date, p63 was reported to be immunohistochemically detectable in basal cells of all squamous epithelia (including epidermis and hair follicles), in basal cells of urothelium, and in basal cells of prostate epithelium11. Generally, UCs are positive for high-molecular-weight cytokeratin (65-100%), p63 (70-92%), thrombomodulin (49-69%), and uroplakin III (57-60%). In carcinomas of unknown primary when differential cytokeratin 7 and 20 staining is used, uriothelial carcinomas are typically cytokeratin 7 positive9,12. On the other side of the coin, adenocarcinoma of lung have been reported that TTF-1 is a more sensitive marker than p6313. In the present case, immunohistochemical analysis showed a biopsy specimen to be positive for cytokeratin 7 and p63 (Figure 2), and negative for TTF-1.
In general, cisplatin-based combination chemotherapy is the standard first-line treatment for patients with metastatic UC8. However, chemotherapy had no effect in our patient. This was probably due to the disease being more aggressive in younger patients6. Additional studies are needed to optimize treatment in patients with metastatic UC. As our case shows, metastatic disease may occur years or even decades after successful treatment of the primary tumor by surgery and adjuvant treatment. It has been proposed that this latency period is due to a clinical phenomenon named tumor dormancy14.
In summary, this case illustrates several distinctive aspects of the care of patients with UUT-UC. First, despite surveillance for 10 years after surgical treatment of low-grade, noninvasive UUT-UC, distant metastases developed later in life without local recurrence. Second, patients younger than 60 years with metastatic UC may have a propensity to aggressive disease. Third, UC has similar histology to adenocarcinoma.
Figures and Tables
Figure 1
Histopathological findings of the biopsied tissue (A-C, H&E stain, ×400). (A) Primary urothelial carcinoma was low grade diffusely involving renal pelvis with subepithelial connective tissue invasion 12 years ago. (B) The tumor of the lung, which was initially suspected as primary lung adenocarcinoma, is revealed as metastatic urothelial carcinoma showing similar histologic features to primary urothelial carcinoma. (C) After chemotherapy, the tracheal tumor cells showing more pleomorphic and anaplastic cytologic features with individual tumor cell infiltration.

References
1. Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011; 59:584–594.
2. Tawfiek ER, Bagley DH. Upper-tract transitional cell carcinoma. Urology. 1997; 50:321–329.
3. Seymour JE, Malin JM Jr, Pierce JM Jr. Late metastases of a superficial transitional cell carcinoma of the bladder: report of a case. J Urol. 1972; 108:277–278.
4. Dougherty DW, Gonsorcik VK, Harpster LE, Trussell JC, Drabick JJ. Superficial bladder cancer metastatic to the lungs: two case reports and review of the literature. Urology. 2009; 73:210.e3–210.e5.
5. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998; 52:594–601.
6. Sengelov L, Kamby C, von der Maase H. Pattern of metastases in relation to characteristics of primary tumor and treatment in patients with disseminated urothelial carcinoma. J Urol. 1996; 155:111–114.
7. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000; 164:1523–1525.
8. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011; 29:2432–2438.
9. Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009; 22:Suppl 2. S96–S118.
10. Nigwekar P, Amin MB. The many faces of urothelial carcinoma: an update with an emphasis on recently described variants. Adv Anat Pathol. 2008; 15:218–233.
11. Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001; 116:823–830.
12. Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol. 2006; 125:675–681.
13. Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol. 2011; 35:15–25.
14. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007; 7:834–846.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download