Abstract
A 73-year-old, previously healthy man presented with nausea, vomiting, diarrhea, dry mouth and febrile sensation 3 hours after eating boiled wild mushrooms. After admission, he showed progressive severe respiratory distress, pancytopenia, azotemia, hypotension, hypoxemia and consolidation of the entire left lung on chest radiography. With a preliminary diagnosis of necrotizing pneumonia, he underwent left pneumonectomy in order to remove all necrotic lung tissue. Lung histology showed extensive hemorrhagic necrosis, massive inflammatory cell infiltration, prominent proliferation of young fibroblasts and the formation of an early-stage hyaline membrane along the alveolar wall. Despite aggressive treatment, including mechanical ventilation, continuous renal replacement therapy and administration of granulocyte colony stimulating factor and broad spectrum antibiotics, he died on hospitalization day 13. Subsequently, the mushroom was identified as Podostroma cornu-damae. This is the first case of a histological evidence of lung involvement by Podostroma cornu-damae poisoning in Korea.
There are about >5,000 species of mushrooms in the world; of which only 30% have been named and classified and only 3% of these are known to be poisonous1. Various types of wild mushrooms that grow in the forests and mountains in Korea are commonly consumed by the locals, especially as traditional and alternative medicines. Therefore, it is very important to discriminate between poisonous and nonpoisonous mushrooms, although previous experience and observation aid in their differentiation2. Recently, a case of fatal poisoning caused by Podostroma cornu-damae ingestion was reported in the Niigata prefecture in Japan3; however, such poisoning is extremely rare in Korea. Herein, we present a case report and literature review of fatal respiratory failure caused by the ingestion of this toxic mushroom.
A 73-year-old man presented with nausea, vomiting, diarrhea, dry mouth, and hot flushes 3 hours after eating boiled wild mushrooms. He visited a primary clinic, where his blood pressure was 70/50 mmHg. Laboratory findings showed azotemia and leukocytosis, and he was referred to the emergency department of our hospital. His wife also experienced nausea and vomiting that resolved spontaneously. He had a history of cigarette smoking (35 packs/yr). His medical history was insignificant, and he had not been on any medication (including over-the-counter medication) and was denied contact with any sick persons, use of illicit drugs, exposure to animals, and recent travel outside Seoul.
On admission, his blood pressure was 106/51 mm Hg, heart rate was 115 beats/min, respiratory rate was 24/min, and body temperature was 37.1℃. He looked acutely ill but was mentally alert. Laboratory tests revealed the following: white blood cell (WBC) count, 34,670/µL (absolute neutrophil count [ANC], 32,243/µL); hemoglobin (Hb), 17.0 g/dL; platelet count, 322,000/µL; blood urea nitrogen, 47.9 mg/dL; serum creatinine, 1.6 mg/dL (reference, 0.6-1.1 mg/dL); total protein, 6.2 g/dL; albumin, 3.7 g/dL; aspartate aminotransferase (AST), 34 IU/L (reference, 5-40 IU/L); alanine aminotransferase (ALT), 19 IU/L (reference, 5-40 IU/L); lactic dehydrogenase (LDH), 313 IU/L (reference, 145-250 IU/L); creatine kinase (CK), 213 IU/L (reference, 38-160 IU/L); serum Na/K/Cl, 140/4.9/108 mEq/L; total CO2, 17.1 mmol/L; serum glucose, 180 mg/dL; HbA1c, 7.3%; C-reactive protein (CRP), 159 mg/dL (reference, 0.0-8.0 mg/dL); and prothrombin time (PT) international normalized ratio (INR), 1.24. After receiving 2 L/min of oxygen via nasal prongs, blood gas analysis revealed the following: pH, 7.351; PCO2, 30.1 mmHg; PO2, 65.4 mmHg; HCO3, 18.2 mmol/L; and SaO2, 91.8%. Chest radiography showed slight blunting of the left costophrenic angle (Figure 1A). Urinalysis revealed no proteinuria or hematuria. After admission, he received a large amount of fluid (9 L over 24 hours), and his clinical condition improved as vomiting and diarrhea had subsided until day 3 of hospitalization.
On day 4, he developed sudden shortness of breath, coughing, and severe respiratory distress. His laboratory examinations were more aggravated as follows: WBC count, 2,840/µL (ANC 2,720/µL); platelet count, 57,000/L; PT INR, 1.16; activated partial thromboplastin time (aPTT), 38.0 seconds; fibrin degradation product >20 µg/mL (reference, 0.0-5.0 µg/mL); plasma D-dimer, 6.56 µg/mL (reference, 0.0-0.5 µg/mL); CRP, 185 mg/L; AST, 82 IU/L; ALT, 50 IU/L; total bilirubin, 0.6 mg/dL; and serum creatinine, 1.6 mg/dL. Chest radiography revealed new widespread consolidations in the whole left lung (Figure 1B). A subsequent computed tomographic (CT)-scan showed extensive consolidations in the left lung and multiple consolidations from the sublobular to subsegmental areas in the right lung, as well as a small amount of pleural effusion in both lungs (Figure 2). However, the CT scan did not show any intra-abdominal infection foci or abnormal findings to explain the severe pneumonia sepsis. Bronchoscopy showed no evidence of endobronchial lesions. He received mechanical ventilation and continuous renal replacement therapy (CRRT) owing to severe hypoxemic respiratory failure, a PaO2/FiO2<200 mm Hg, and acute oliguric kidney failure. However, he developed refractory shock and his condition rapidly deteriorated. We suspected a possible fulminant course of necrotizing pneumonia and considered surgical intervention and thus consulted the thoracic department. He underwent left pneumonectomy because the gross appearance of the lung inflammation suggested impending pulmonary gangrene. Skin lesions such as miliaria, bullae, and erythroderma were found on the chest wall, face, and upper extremities (not shown). Lung histology revealed varying stages of inflammation with areas of extensive hemorrhagic necrosis (Figure 3A), massive inflammatory cell infiltration and edema in the alveolar space and interstitium (Figure 3B), type II pneumocyte hyperplasia and prominent proliferation of young fibroblasts (Figure 3C), and the formation of a hyaline membrane along the alveolar wall (Figure 3D).
He subsequently suffered from rhabdomyolysis, kidney failure, hepatic failure, pancytopenia, disseminated intravascular coagulopathy, and prolonged shock. On day 7, his body temperature was 39.4℃, and his laboratory examinations were more aggravated as follows: WBC count, 120/µL (ANC, 80/µL); platelet count, 32,000/µL; PT INR, 1.50; aPTT, 41.7 seconds; fibrin degradation product>20 µg/mL (reference, 0.0-5.0 µg/mL); plasma D-dimer, 4.95 µg/mL (reference, 0.0-0.5 µg/mL); CRP, 250 mg/L; AST, 122 IU/L; ALT, 54 IU/L; total bilirubin, 5.0 mg/dL; serum creatinine, 2.0 mg/dL; LDH, 500 IU/L; CK, 4,997 IU/L; and serum myoglobin>1,000 ng/mL (reference, 10-92 ng/mL). A peripheral blood smear showed severe pancytopenia mimicking aplastic anemia. Treatment comprising fluid resuscitation with crystalloid and inotropics was performed. Although causative infectious pathogens were not identified from bronchial washing or blood and lung tissues, broad-spectrum antibiotics such as carbapenem, vancomycin, and respiratory quinolone were administrated. Despite the administration of granulocyte colony stimulating factor (GCSF), transfusion of platelet concentrates and packed red blood cells, high doses of inotropics (including norepinephrine, dopamine, and dobutamine), as well as lung protective ventilation strategy and CRRT, he did not recover from multiorgan failure and died on day 13 of hospitalization.
The wild mushroom specimen in question was sent to a team of expert mycologists, at the National Academy of Agricultural Science in Korea, were it was determined to be P. cornu-damae (Figure 4).
P. cornu-damae is a species of fungus in the Hypocreaceae family, originally described as Hypocrea cornu-damae in 1895 and later transferred to the genus Podocrea in 1905. In 1994, it was renamed Podostroma by Japanese mycologists4,5. It is shaped like a bright red coral or the red horn of a deer species found in the mountains and forests of Korea (Figure 4B). This fungus is unfamiliar to the Korean population. However, several cases of poisoning from P. cornu-damae have been reported in Japan; 2 fatalities 2 days after the consumption of 1.0 g of the mushroom soaked in alcohol, and 1, after the consumption of the fried mushroom6. Thus, the local Japanese population has been warned to be wary of consuming this mushroom.
Till date, there was only one report of P. cornu-damae poisoning in Korea7; of the 2 people who ate the boiled fungus, 1 died due to multi-organ failure despite aggressive medical therapy, and the other improved after conservative management for 1 month. Symptoms associated with consumption in those cases included chills, high fever, generalized weakness, headache, sore throat, hypotension, and desquamation on the palms and soles. Laboratory findings in the lethal case revealed severe refractory pancytopenia and multi-organ failure. In other instances, consumption of this fungus resulted in gastrointestinal disorders, erroneous perception, decrease in the number of leukocytes and thrombocytes, peeling skin on the face, loss of hair, and atrophy of the cerebellum, leading to speech disturbance and voluntary movement disorder6. In another fatal case, autopsy revealed multiple organ failure, including acute kidney failure, liver necrosis, and bone marrow failure, as well as disseminated intravascular coagulation8,9.
In this report, a 73-year-old man initially presented with nausea, vomiting, diarrhea, and skin lesions few hours after consuming the wild mushroom, which was followed by severe hypoxemic respiratory failure, hypotension, oliguria, leucopenia, thrombocytopenia, and disseminated intravascular coagulation. Without rapid correction of these problems, multiorgan failure ensues within a short time and turns fatal. Similar to previously reported cases, this patient did not respond to currently available conventional medical therapy, including lung protective ventilation strategy, early CRRT10, and the use of broad spectrum antibiotics, GCSF, and high doses of inotropics. Thus, this fungus can be classified as a very toxic mushroom with a lethal effect on humans. Saikawa et al.6 reported that roridin E, satratoxin H, satratoxin H 12',13'-diacetate, satratoxin H 12'-acetate, and satratoxin H 13'-acetate isolated from the mushroom P. cornu-damae had a lethal effect on mice, observed just 1 day after injection. However, lung involvement caused by ingestion of P. cornu-damae is not documented. In this case, the patient's condition rapidly deteriorated suggesting not only fulminant necrotizing pneumonia, but also pulmonary gangrene. Thus, surgery to remove the necrotic tissue was performed, since resection of all gangrenous tissue is mandatory and is life saving in case of pulmonary gangrene11. The patient's left lung showed extensive hemorrhagic necrosis, massive pneumonitis, fibrin exudates, and hyaline membrane formation, but no evidence of any microorganisms. These histologic findings may have extended to other important organs, including the kidney, liver, and bone marrow. Unfortunately, there are currently no therapeutic modalities to restore these pathologic processes to prevent organ failure and death.
Mushroom poisoning is a public health problem all over the world9. Physicians as well as health care providers should be aware of P. cornu-damae poisoning because it can be fatal, despite treatment. Consumption of raw wild mushrooms should be prohibited.
Figures and Tables
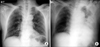 | Figure 1(A) Chest radiography at admission shows slight costophrenic angle blunting. (B) The consolidations are rapidly more aggravated in two-thirds of the left lung on hospital day 4. |
 | Figure 2Chest computed tomography on hospital day 4 shows extensive consolidation in the left lung, multiple consolidations in the sublobular to subsegmental areas in the right lung, and a small amount of pleural effusion in both lungs. |
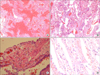 | Figure 3Variable lung histology associated with Podostroma cornu-damae poisoning. Extensive hemorrhagic necrosis (A; H&E stain, ×40), massive inflammatory cell infiltration causing interstitial pneumonitis (B; H&E stain, ×200), type II pneumocyte hyperplasia, prominent proliferation of young fibroblasts, and sprouting alveolar lining cells (C; H&E stain, ×400); in addition, fibrin exudates and hyaline membrane formation (D; H&E stain, ×400) were seen. |
References
1. Gonmori K, Yoshioka N, et al. The examination of mushroom poisonings at Akita University. Leg Med (Tokyo). 2003; 5 Suppl 1:S83–S86.
2. Faulstich H. Mushroom poisoning. Lancet. 1980; 2:794–795.
3. Yokoyama K, Gonmori K. Increase of poisoning by tropical mushrooms in Japan in recent years. Chudoku Kenkyu. 2009; 22:240–248.
4. Izawa H. Podostroma cornu-damae (Pat.). Mycobank International Mycological Association;1994.
5. Patouillard NT. Enumération des champignons récoltés par les RR. PP. Farges et Soulié, dans le Thibet oriental et le Sutchuen. Bull Soc Mycol Fr. 1895; 11:196–199.
6. Saikawa Y, Okamoto H, Inui T, Makabe M, Okuno T, Suda T, et al. Toxic principles of a poisonous mushroom Podostroma cornu-damae. Tetrahedron. 2001; 57:8277–8281.
7. Ahn JY, Seok SJ, Song JE, Choi JH, Han SH, Choi JY, et al. Two cases of mushroom poisoning by Podostroma cornu-damae. Yonsei Med J. 2013; 54:265–268.
8. Mogi K, Takeshita H, Yasuda T, Nakajo S, Koichiro K. Case report: food poisoning to death by Podostroma cornu-damae, its case history and autopsy findings. Acta Criminol Med Leg Jpn. 2003; 69:14–20.
9. Eren SH, Demirel Y, Ugurlu S, Korkmaz I, Aktas C, Guven FM. Mushroom poisoning: retrospective analysis of 294 cases. Clinics (Sao Paulo). 2010; 65:491–496.
10. Suzuki M, Katoh Y, Kumagai H, Saitoh M, Ishikawa H, Itoh H, et al. Successful treatment in a case of podostroma cornu-damae poisoning, a deadly poisonous mushroom. Chudoku Kenkyu. 2002; 15:177–182.
11. Refaely Y, Weissberg D. Gangrene of the lung: treatment in two stages. Ann Thorac Surg. 1997; 64:970–973.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download